Virological efficacy of PI monotherapy for HIV-1 in clinical practice
- PMID: 27402006
- PMCID: PMC5079296
- DOI: 10.1093/jac/dkw265
Virological efficacy of PI monotherapy for HIV-1 in clinical practice
Abstract
Background: Clinical trials of PI monotherapy indicate that most participants maintain viral suppression and emergent protease resistance is rare. However, outcomes among patients receiving PI monotherapy for clinical reasons, such as toxicity or adherence issues, are less well studied.
Methods: An observational study of patients attending an HIV treatment centre in London, UK, who had received PI monotherapy between 2004 and 2013, was conducted using prospectively collected clinical data and genotypic resistance reports. Survival analysis techniques were used to examine the times to virological failure and treatment discontinuation.
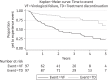
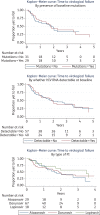
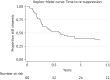
Results: Ninety-five patients had PI monotherapy treatment for a median duration of 126 weeks. Virological failure occurred during 64% of episodes and 8% of patients developed emergent protease mutations. We estimate failure occurs in half of episodes within 2 years following initiation. Where PI monotherapy was continued following virological failure, 68% of patients achieved viral re-suppression. Despite a high incidence of virological failure, many patients continued PI monotherapy and 79% of episodes were ongoing at the end of the study. The type of PI used, the presence of baseline protease mutations and the plasma HIV RNA at initiation did not have a significant impact on treatment outcomes.
Conclusions: There was a higher incidence of virological failure and emerging resistance in our UK clinical setting than described in PI monotherapy clinical trials and other European observational studies. Despite this, many patients continued PI monotherapy and regained viral suppression, indicating this strategy remains a viable option in certain individuals following careful clinical evaluation.
© The Author 2016. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
Similar articles
-
The Protease Inhibitor Monotherapy Versus Ongoing Triple Therapy (PIVOT) trial: a randomised controlled trial of a protease inhibitor monotherapy strategy for long-term management of human immunodeficiency virus infection.Health Technol Assess. 2016 Mar;20(21):1-158. doi: 10.3310/hta20210. Health Technol Assess. 2016. PMID: 26986803 Free PMC article. Clinical Trial.
-
Effectiveness of Ritonavir-Boosted Protease Inhibitor Monotherapy in Clinical Practice Even with Previous Virological Failures to Protease Inhibitor-Based Regimens.PLoS One. 2016 Feb 12;11(2):e0148924. doi: 10.1371/journal.pone.0148924. eCollection 2016. PLoS One. 2016. PMID: 26872331 Free PMC article.
-
Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial.Lancet HIV. 2015 Oct;2(10):e417-26. doi: 10.1016/S2352-3018(15)00176-9. Epub 2015 Sep 14. Lancet HIV. 2015. PMID: 26423649 Free PMC article. Clinical Trial.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
Cited by
-
Sustained viral suppression with dolutegravir monotherapy in a treatment-experienced adult with perinatally acquired HIV.BMJ Case Rep. 2021 Nov 2;14(11):e243685. doi: 10.1136/bcr-2021-243685. BMJ Case Rep. 2021. PMID: 34728501 Free PMC article.
-
Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a Phase IIa randomised controlled study.J Virus Erad. 2019 Jan 1;5(1):10-22. doi: 10.1016/S2055-6640(20)30273-9. J Virus Erad. 2019. PMID: 30800421 Free PMC article.
-
Diffuse White Matter Signal Abnormalities on Magnetic Resonance Imaging Are Associated With Human Immunodeficiency Virus Type 1 Viral Escape in the Central Nervous System Among Patients With Neurological Symptoms.Clin Infect Dis. 2017 Apr 15;64(8):1059-1065. doi: 10.1093/cid/cix035. Clin Infect Dis. 2017. PMID: 28329096 Free PMC article.
-
Observational study to evaluate discontinuation of monotherapy with cobicistat-boosted darunavir in patients with human immunodeficiency virus.Medicine (Baltimore). 2022 Dec 9;101(49):e32208. doi: 10.1097/MD.0000000000032208. Medicine (Baltimore). 2022. PMID: 36626459 Free PMC article.
-
HIV Cerebrospinal Fluid Escape and Neurocognitive Pathology in the Era of Combined Antiretroviral Therapy: What Lies Beneath the Tip of the Iceberg in Sub-Saharan Africa?Brain Sci. 2018 Oct 20;8(10):190. doi: 10.3390/brainsci8100190. Brain Sci. 2018. PMID: 30347806 Free PMC article. Review.
References
-
- Hill A, McBride A, Sawyer AW et al. . Resistance at virological failure using boosted protease inhibitors versus nonnucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy—implications for sustained efficacy of ART in resource-limited settings. J Infect Dis 2013; 207 Suppl 2: S78–84. - PubMed
-
- WHO. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

