Positive regulation of the Candida albicans multidrug efflux pump Cdr1p function by phosphorylation of its N-terminal extension
- PMID: 27402010
- PMCID: PMC5079294
- DOI: 10.1093/jac/dkw252
Positive regulation of the Candida albicans multidrug efflux pump Cdr1p function by phosphorylation of its N-terminal extension
Abstract
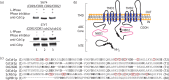
Objectives: Overexpression of ATP-binding cassette (ABC) transporters is a frequent cause of multidrug resistance in cancer cells and pathogenic microorganisms. One example is the Cdr1p transporter from the human fungal pathogen Candida albicans that belongs to the pleiotropic drug resistance (PDR) subfamily of ABC transporters found in fungi and plants. Cdr1p is overexpressed in several azole-resistant clinical isolates, causing azole efflux and treatment failure. Cdr1p appears as a doublet band in western blot analyses, suggesting that the protein is post-translationally modified. We investigated whether Cdr1p is phosphorylated and the function of this modification.
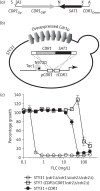
Methods: Phosphorylated residues were identified by MS. Their function was investigated by site-directed mutagenesis and expression of the mutants in a C. albicans endogenous system that exploits a hyperactive allele of the Tac1p transcription factor to drive high levels of Cdr1p expression. Fluconazole resistance was measured by microtitre plate and spot assays and transport activity by Nile red accumulation.
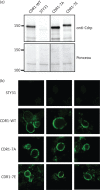
Results: We identified a cluster of seven phosphorylated amino acids in the N-terminal extension (NTE) of Cdr1p. Mutating all seven sites to alanine dramatically diminished the ability of Cdr1p to confer fluconazole resistance and transport Nile red, without affecting Cdr1p localization. Conversely, a Cdr1p mutant in which the seven amino acids were replaced by glutamate was able to confer high levels of fluconazole resistance and to export Nile red.
Conclusions: Our results demonstrate that the NTE of Cdr1p is phosphorylated and that NTE phosphorylation plays a major role in regulating Cdr1p and possibly other PDR transporter function.
© The Author 2016. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance.J Antimicrob Chemother. 2004 Dec;54(6):999-1006. doi: 10.1093/jac/dkh456. Epub 2004 Oct 14. J Antimicrob Chemother. 2004. PMID: 15486081
-
ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates.Antimicrob Agents Chemother. 2008 Nov;52(11):3851-62. doi: 10.1128/AAC.00463-08. Epub 2008 Aug 18. Antimicrob Agents Chemother. 2008. PMID: 18710914 Free PMC article.
-
Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters.Antimicrob Agents Chemother. 2001 Dec;45(12):3366-74. doi: 10.1128/AAC.45.12.3366-3374.2001. Antimicrob Agents Chemother. 2001. PMID: 11709310 Free PMC article.
-
Candida Efflux ATPases and Antiporters in Clinical Drug Resistance.Adv Exp Med Biol. 2016;892:351-376. doi: 10.1007/978-3-319-25304-6_15. Adv Exp Med Biol. 2016. PMID: 26721282 Review.
-
Yeast ATP-binding cassette transporters conferring multidrug resistance.Annu Rev Microbiol. 2012;66:39-63. doi: 10.1146/annurev-micro-092611-150111. Epub 2012 Jun 11. Annu Rev Microbiol. 2012. PMID: 22703054 Review.
Cited by
-
The Set1 Histone H3K4 Methyltransferase Contributes to Azole Susceptibility in a Species-Specific Manner by Differentially Altering the Expression of Drug Efflux Pumps and the Ergosterol Gene Pathway.Antimicrob Agents Chemother. 2022 May 17;66(5):e0225021. doi: 10.1128/aac.02250-21. Epub 2022 Apr 26. Antimicrob Agents Chemother. 2022. PMID: 35471041 Free PMC article.
-
The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy.Front Pharmacol. 2017 Jan 10;7:535. doi: 10.3389/fphar.2016.00535. eCollection 2016. Front Pharmacol. 2017. PMID: 28119610 Free PMC article. Review.
-
The global regulator Ncb2 escapes from the core promoter and impacts transcription in response to drug stress in Candida albicans.Sci Rep. 2017 Apr 6;7:46084. doi: 10.1038/srep46084. Sci Rep. 2017. PMID: 28383050 Free PMC article.
-
Functional analysis of Candida albicans Cdr1 through homologous and heterologous expression studies.FEMS Yeast Res. 2025 Jan 30;25:foaf012. doi: 10.1093/femsyr/foaf012. FEMS Yeast Res. 2025. PMID: 40101948 Free PMC article. Review.
-
Vacuolar Sequestration of Azoles, a Novel Strategy of Azole Antifungal Resistance Conserved across Pathogenic and Nonpathogenic Yeast.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01347-18. doi: 10.1128/AAC.01347-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30642932 Free PMC article.
References
-
- Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet 2005; 6: 123–42. - PubMed
-
- Jones PM, O'Mara ML, George AM. ABC transporters: a riddle wrapped in a mystery inside an enigma. Trends Biochem Sci 2009; 34: 520–31. - PubMed
-
- Dean M. The genetics of ATP-binding cassette transporters. Meth Enzymol 2005; 400: 409–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

