Global Epidemiology of Plasmodium vivax
- PMID: 27402513
- PMCID: PMC5198891
- DOI: 10.4269/ajtmh.16-0141
Global Epidemiology of Plasmodium vivax
Abstract
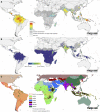
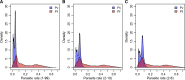
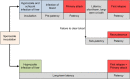
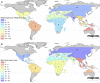
Plasmodium vivax is the most widespread human malaria, putting 2.5 billion people at risk of infection. Its unique biological and epidemiological characteristics pose challenges to control strategies that have been principally targeted against Plasmodium falciparum Unlike P. falciparum, P. vivax infections have typically low blood-stage parasitemia with gametocytes emerging before illness manifests, and dormant liver stages causing relapses. These traits affect both its geographic distribution and transmission patterns. Asymptomatic infections, high-risk groups, and resulting case burdens are described in this review. Despite relatively low prevalence measurements and parasitemia levels, along with high proportions of asymptomatic cases, this parasite is not benign. Plasmodium vivax can be associated with severe and even fatal illness. Spreading resistance to chloroquine against the acute attack, and the operational inadequacy of primaquine against the multiple attacks of relapse, exacerbates the risk of poor outcomes among the tens of millions suffering from infection each year. Without strategies accounting for these P. vivax-specific characteristics, progress toward elimination of endemic malaria transmission will be substantially impeded.
© The American Society of Tropical Medicine and Hygiene.
Figures


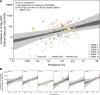
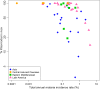
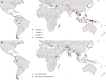
References
-
- PATH . Staying the Course? Malaria Research and Development in a Time of Economic Uncertainty. Seattle, WA: PATH; 2011.
-
- Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol. 2013;81:77–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

