Differential regulation of autophagy and mitophagy in pulmonary diseases
- PMID: 27402690
- PMCID: PMC5504426
- DOI: 10.1152/ajplung.00128.2016
Differential regulation of autophagy and mitophagy in pulmonary diseases
Abstract
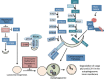
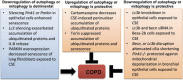
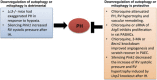
Lysosomal-mediated degradation of intracellular lipids, proteins and organelles, known as autophagy, represents a inducible adaptive response to lung injury resulting from exposure to insults, such as hypoxia, microbes, inflammation, ischemia-reperfusion, pharmaceuticals (e.g., bleomycin), or inhaled xenobiotics (i.e., air pollution, cigarette smoke). This process clears damaged or toxic cellular constituents and facilitates cell survival in stressful environments. Autophagic degradation of dysfunctional or damaged mitochondria is termed mitophagy. Enhanced mitophagy is usually an early response to promote survival. However, overwhelming or prolonged mitochondrial damage can induce excessive/pathological levels of mitophagy, thereby promoting cell death and tissue injury. Autophagy/mitophagy is therefore an important modulator in human pulmonary diseases and a potential therapeutic target. This review article will summarize the most recent studies highlighting the role of autophagy/mitophagy and its molecular pathways involved in stress response in pulmonary pathologies.
Keywords: ALI; COPD; IPF; PAH; PH.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Aguirre A, Lopez-Alonso I, Gonzalez-Lopez A, Amado-Rodriguez L, Batalla-Solis E, Astudillo A, Blazquez-Prieto J, Fernandez AF, Galvan JA, dos Santos CC, Albaiceta GM. Defective autophagy impairs ATF3 activity and worsens lung injury during endotoxemia. J Mol Med 92: 665–676, 2014. - PubMed
-
- Ahuja S, Knudsen L, Chillappagari S, Henneke I, Ruppert C, Korfei M, Gochuico BR, Bellusci S, Seeger W, Ochs M, Guenther A, Mahavadi P. MAP1LC3B overexpression protects against Hermansky-Pudlak syndrome type-1 induced defective autophagy in vitro. Am J Physiol Lung Cell Mol Physiol 310: L519–L531, 2016. - PMC - PubMed
-
- Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L56–L69, 2013. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

