Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke
- PMID: 27402738
- PMCID: PMC4968743
- DOI: 10.1073/pnas.1521083113
Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke
Abstract
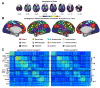
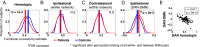
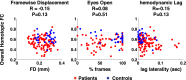
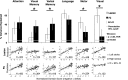
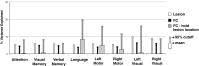
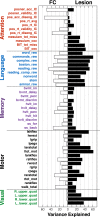
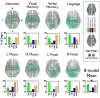
Deficits following stroke are classically attributed to focal damage, but recent evidence suggests a key role of distributed brain network disruption. We measured resting functional connectivity (FC), lesion topography, and behavior in multiple domains (attention, visual memory, verbal memory, language, motor, and visual) in a cohort of 132 stroke patients, and used machine-learning models to predict neurological impairment in individual subjects. We found that visual memory and verbal memory were better predicted by FC, whereas visual and motor impairments were better predicted by lesion topography. Attention and language deficits were well predicted by both. Next, we identified a general pattern of physiological network dysfunction consisting of decrease of interhemispheric integration and intrahemispheric segregation, which strongly related to behavioral impairment in multiple domains. Network-specific patterns of dysfunction predicted specific behavioral deficits, and loss of interhemispheric communication across a set of regions was associated with impairment across multiple behavioral domains. These results link key organizational features of brain networks to brain-behavior relationships in stroke.
Keywords: functional connectivity; interhemispheric; language; memory; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Carrera E, Tononi G. Diaschisis: Past, present, future. Brain. 2014;137(Pt 9):2408–2422. - PubMed
-
- von Monakow C. 1914. Die Lokalisation im Grosshirn: und der Abbau der Funktion durch kortikale Herde [Localization in the Cerebrum and the Degeneration of Functions Through Conical Sources] (Bergmann, Wiesbaden, Germany). German.
-
- Corbetta M. Functional connectivity and neurological recovery. Dev Psychobiol. 2012;54(3):239–253. - PubMed
-
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16(3):159–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

