Niemann-Pick type C2 deficiency impairs autophagy-lysosomal activity, mitochondrial function, and TLR signaling in adipocytes
- PMID: 27402802
- PMCID: PMC5003158
- DOI: 10.1194/jlr.M066522
Niemann-Pick type C2 deficiency impairs autophagy-lysosomal activity, mitochondrial function, and TLR signaling in adipocytes
Abstract
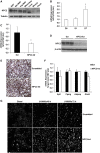
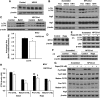
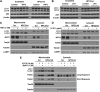
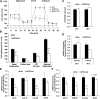
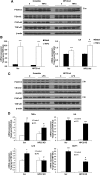
In this study, we investigated the role and mechanism of Niemann-Pick type C (NPC)2 in regulating lysosomal activity, mitophagy, and mitochondrial function in adipocytes. We found that knocking down NPC2 impaired lysosomal activity, as evidenced by the reduced mature cathepsin B, the increased accumulation of light chain 3 (LC3) and p62, and the decreased autophagic flux. In NPC2-knockdown (kd) adipocytes, the starvation-induced conversion of LC3-I to LC3-II was abolished. More interestingly, the majority of NPC2 was found in the mitochondrial fraction, and NPC2 deficiency led to impaired autophagic flux and decreased induction of LC3-II in the mitochondrial fraction during mitochondrial stress. Moreover, cellular respiration profiling revealed that NPC2-kd adipocytes had significantly decreased basal/maximal respiration and mitochondrial gene expression compared with scrambled cells, suggesting mitochondrial dysfunction. Additionally, we found that the mitochondrial recruitment of LC3-II induced by lipopolysaccharide (LPS), but not TNFα, was blunted in NPC2-kd adipocytes. Most intriguingly, NPC2-kd selectively diminished LPS-induced NFκB and ERK1/2 phosphorylation and the expression of pro-inflammatory genes, indicating that toll-like receptor signaling activation is impaired in the absence of NPC2. Our results suggest that NPC2 is in a mitochondrially associated autophagosome and plays an important role in regulating mitophagy, mitochondrial quality control, and mitochondrial function.
Keywords: Niemann-Pick disease; inflammation; mitochondria; obesity • lysosome; toll-like receptor.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Lionaki E., Markaki M., Palikaras K., and Tavernarakis N.. 2015. Mitochondria, autophagy and age-associated neurodegenerative diseases: new insights into a complex interplay. Biochim. Biophys. Acta. 1847: 1412–1423. - PubMed
-
- Mizushima N., and Komatsu M.. 2011. Autophagy: renovation of cells and tissues. Cell. 147: 728–741. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

