Estimating (131)I biokinetics and radiation doses to the red marrow and whole body in thyroid cancer patients: probe detection versus image quantification
- PMID: 27403014
- PMCID: PMC4938444
- DOI: 10.1590/0100-3984.2015.0079
Estimating (131)I biokinetics and radiation doses to the red marrow and whole body in thyroid cancer patients: probe detection versus image quantification
Abstract
Objective: To compare the probe detection method with the image quantification method when estimating (131)I biokinetics and radiation doses to the red marrow and whole body in the treatment of thyroid cancer patients.
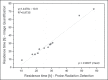
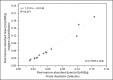
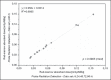
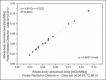
Materials and methods: Fourteen patients with metastatic thyroid cancer, without metastatic bone involvement, were submitted to therapy planning in order to tailor the therapeutic amount of (131)I to each individual. Whole-body scans and probe measurements were performed at 4, 24, 48, 72, and 96 h after (131)I administration in order to estimate the effective half-life (Teff) and residence time of (131)I in the body.
Results: The mean values for Teff and residence time, respectively, were 19 ± 9 h and 28 ± 12 h for probe detection, compared with 20 ± 13 h and 29 ± 18 h for image quantification. The average dose to the red marrow and whole body, respectively, was 0.061 ± 0.041 mGy/MBq and 0.073 ± 0.040 mGy/MBq for probe detection, compared with 0.066 ± 0.055 mGy/MBq and 0.078 ± 0.056 mGy/MBq for image quantification. Statistical analysis proved that there were no significant differences between the two methods for estimating the Teff (p = 0.801), residence time (p = 0.801), dose to the red marrow (p = 0.708), and dose to the whole body (p = 0.811), even when we considered an optimized approach for calculating doses only at 4 h and 96 h after (131)I administration (p > 0.914).
Conclusion: There is full agreement as to the feasibility of using probe detection and image quantification when estimating (131)I biokinetics and red-marrow/whole-body doses. However, because the probe detection method is inefficacious in identifying tumor sites and critical organs during radionuclide therapy and therefore liable to skew adjustment of the amount of (131)I to be administered to patients under such therapy, it should be used with caution.
Objetivo: Comparar o desempenho dos métodos de detecção de sonda e quantificação de imagens na estimativa da biocinética do radioisótopo 131I e das doses de radiação na medula óssea vermelha e no corpo inteiro durante a radioiodoterapia em pacientes com câncer de tireoide.
Materiais e métodos: Catorze pacientes portadores de câncer metastático de tireoide, sem acometimento ósseo, foram submetidos ao planejamento terapêutico visando estabelecer a melhor atividade de 131I a ser empregada na radioiodoterapia. Imagens cintilográficas e captações de corpo inteiro foram adquiridas 4, 24, 48, 72 e 96 h após a administração de atividades traçadoras de 131I, visando estimar a meia-vida efetiva (T1/2ef) e o tempo de residência do 131I no organismo dos pacientes.
Resultados: Os valores médios de T1/2ef e tempo de residência foram, respectivamente, 19 ± 9 h e 28 ± 12 h pelo método de detecção de sonda e 20 ± 13 h e 29 ± 18 h pela quantificação de imagens. As doses médias na medula óssea vermelha e no corpo inteiro foram, respectivamente, 0,061 ± 0,041 mGy/MBq e 0,073 ± 0,040 mGy/MBq pelo método de detecção de sonda e 0,066 ± 0,055 mGy/MBq e 0,078 ± 0,056 mGy/MBq pela quantificação de imagens. A análise estatística demonstrou que os dois métodos apresentam desempenho semelhante no tocante à estimativa de T1/2ef (p = 0,801), tempo de residência (p = 0,801) e doses, tanto na medula óssea vermelha (p = 0,708) como no corpo inteiro (p = 0,811), mesmo com métodos otimizados de dosimetria que levam em consideração somente dois pontos de medida (4 h e 96 h) após a administração de 131I (p > 0,914).
Conclusão: Existe excelente concordância entre o método de detecção de sonda e a quantificação de imagens quanto à estimativa da biocinética do 131I e das doses absorvidas de radiação. Contudo, o método de detecção de sonda deve ser usado com cuidado por ser incapaz de identificar regiões metastáticas e órgãos críticos durante a terapia com radionuclídeos, podendo distorcer ajustes da atividade de 131I a ser administrada durante a radioiodoterapia.
Keywords: Dosimetry; Iodine radioisotopes/therapeutic use; Radioisotopes/pharmacokinetics; Radiotherapy; Thyroid neoplasms.
Figures







Similar articles
-
Prediction of iodine-131 biokinetics and radiation doses from therapy on the basis of tracer studies: an important question for therapy planning in nuclear medicine.Nucl Med Commun. 2016 May;37(5):473-9. doi: 10.1097/MNM.0000000000000465. Nucl Med Commun. 2016. PMID: 26671852
-
Recombinant Human Thyroid-Stimulating Hormone Versus Thyroid Hormone Withdrawal in 124I PET/CT-Based Dosimetry for 131I Therapy of Metastatic Differentiated Thyroid Cancer.J Nucl Med. 2017 Jul;58(7):1146-1154. doi: 10.2967/jnumed.116.179366. Epub 2017 Jan 19. J Nucl Med. 2017. PMID: 28104741 Free PMC article.
-
Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal.J Nucl Med. 2006 Apr;47(4):648-54. J Nucl Med. 2006. PMID: 16595499 Clinical Trial.
-
Dosimetry of 188Re-hydroxyethylidene diphosphonate in human prostate cancer skeletal metastases.J Nucl Med. 2003 Jun;44(6):953-60. J Nucl Med. 2003. PMID: 12791825 Clinical Trial.
-
PET/CT-derived whole-body and bone marrow dosimetry of 89Zr-cetuximab.J Nucl Med. 2015 Feb;56(2):249-54. doi: 10.2967/jnumed.114.147819. Epub 2015 Jan 22. J Nucl Med. 2015. PMID: 25613538
Cited by
-
Determination of effective half-life of 131I in patients with differentiated thyroid carcinoma: comparison of cystatin C and creatinine-based estimation of renal function.Endocrine. 2019 Mar;63(3):554-562. doi: 10.1007/s12020-018-1800-4. Epub 2018 Nov 1. Endocrine. 2019. PMID: 30382554
-
Very-Low-Dose Radiation and Clinical Molecular Nuclear Medicine.Life (Basel). 2022 Jun 17;12(6):912. doi: 10.3390/life12060912. Life (Basel). 2022. PMID: 35743943 Free PMC article. Review.
-
A practical method of I-131 thyroid cancer therapy dose optimization using estimated effective renal clearance.SAGE Open Med Case Rep. 2017 Dec 7;5:2050313X17745203. doi: 10.1177/2050313X17745203. eCollection 2017. SAGE Open Med Case Rep. 2017. PMID: 29242746 Free PMC article.
-
Red Marrow Absorbed Dose Calculation in Thyroid Cancer Patient Using a Simplified Excel Spreadsheet.Mol Imaging Radionucl Ther. 2020 Oct 19;29(3):124-131. doi: 10.4274/mirt.galenos.2020.71473. Mol Imaging Radionucl Ther. 2020. PMID: 33094576 Free PMC article.
References
-
- Luster M, Clarke SE, Dietlein M, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–1959. - PubMed
-
- Dorn R, Kopp J, Vogt H, et al. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med. 2003;44:451–456. - PubMed
-
- Lassmann M, Hänscheid H, Chiesa C, et al. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur J Nucl Med Mol Imaging. 2008;35:1405–1412. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
