Cardiac Implantable Electronic Device Infection in Patients at Risk
- PMID: 27403296
- PMCID: PMC4939310
- DOI: 10.15420/aer.2015.27.2
Cardiac Implantable Electronic Device Infection in Patients at Risk
Abstract
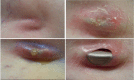
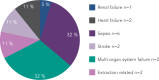
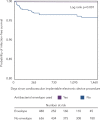
The incidence of infection following implantation of cardiac implantable electronic devices (CIEDs) is increasing at a faster rate than that of device implantation. Patients with a CIED infection usually require hospitalisation and complete device and lead removal. A significant proportion die from their infection. Transvenous lead extraction (TLE) is associated with rare but serious complications including major vascular injury or cardiac perforation. Operator experience and advances in lead extraction methods, including laser technology and rotational sheaths, have resulted in procedures having a low risk of complication and mortality. Strategies for preventing CIED infections include intravenous antibiotics and aseptic surgical techniques. An additional method to reduce CIED infection may be the use of antibacterial TYRX™ envelope. Data from non-randomised cohort studies have indicated that antibacterial envelope use can reduce the incidence of CIED infection by more than 80 % in high-risk patients and a randomised clinical trial is ongoing.
Keywords: Cardiovascular implantable electronic device infections; antibacterial envelope; implantable cardioverter-defibrillators; pacemaker; transvenous lead extraction.
Figures


References
-
- Beck H, Boden WE, Patibandla S, et al. 50th anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am J Cardiol. 2010;106:810–8. PMID: 21391322. - PubMed
-
- Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–6. DOI: 10.1016/j. jacc.2011.04.033; PMID: 21867833. - PubMed
-
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 – a World Society of Arrhythmia’s project, Pacing Clin Electrophysiol. 2011;34:1013–27. DOI: 10.1111/j.1540 8159.2011.03150.x; PMID: 21707667. - PubMed
-
- Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J. 2004;147:582–6. PMID: 15077071. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

