The role for protein tyrosine phosphatase non-receptor type 22 in regulating intestinal homeostasis
- PMID: 27403297
- PMCID: PMC4924425
- DOI: 10.1177/2050640615600115
The role for protein tyrosine phosphatase non-receptor type 22 in regulating intestinal homeostasis
Abstract
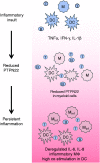
Inflammatory bowel disease represents a chronic intestinal inflammation. Recent knowledge suggests a crucial role for genetic, immunological and bacterial factors in inflammatory bowel disease pathogenesis. Variations within the gene locus encoding PTPN22 have been associated with inflammatory bowel disease. PTPN22 is critically involved in controlling immune cell activation and thereby plays an important role in maintaining intestinal homeostasis. Although in B and T cells the mechanism showing how PTPN22 affects cell signalling pathways is well studied, its role in myeloid cells remains less defined. Regulation of the innate immune system plays an essential role in the intestine, and levels of PTPN22 in myeloid cells are drastically reduced in the intestine of inflammatory bowel disease patients. Therefore, additional studies to define the role of PTPN22 in myeloid cells might clearly enhance our understanding of how PTPN22 contributes to intestinal homeostasis.
Keywords: Crohn’s disease; Inflammatory bowel disease; PTPN22; adaptive immunity; innate immunity; ulcerative colitis.
Figures


References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007; 448: 427–434. - PubMed
-
- Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: Common pathways with other diseases. Gut 2011; 60: 1739–1753. - PubMed
-
- Geremia A, Biancheri P, Allan P, et al. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 2014; 13: 3–10. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

