The combination of oligo- and polysaccharides and reticulated protein for the control of symptoms in patients with irritable bowel syndrome: Results of a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial
- PMID: 27403313
- PMCID: PMC4924437
- DOI: 10.1177/2050640615615050
The combination of oligo- and polysaccharides and reticulated protein for the control of symptoms in patients with irritable bowel syndrome: Results of a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial
Abstract
Background: A medical device containing the film-forming agent reticulated protein and a prebiotic mixture of vegetable oligo- and polysaccharides has been developed, recently receiving European approval as MED class III for the treatment of chronic/functional or recidivant diarrhoea due to different causes including irritable bowel syndrome (IBS). In the present paper, we evaluate a protein preparation containing these components in comparison with placebo in adult patients with diarrhoea-predominant IBS.
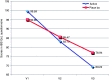
Methods: In a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial, patients were randomly assigned to receive the combination of oligo- and polysaccharides and reticulated protein and placebo (four oral tablets/day for 56 days). Demographic, clinical and quality of life characteristics and presence and intensity of abdominal pain and flatulence (seven-point Likert scale) were assessed at three study visits (baseline and at 28 and 56 days). Stool emissions were recorded on the diary card using the seven-point Bristol Stool Scale.
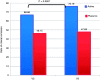
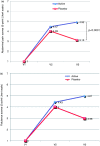
Results: A total of 128 patients were randomised to receive either tablets containing the combination (n = 63) or placebo (n = 65). Treatment with oligo- and polysaccharides and reticulated protein was safe and well tolerated. A significant improvement in symptoms across the study was observed in patients treated with oligo- and polysaccharides and reticulated protein between visit 2 and visit 3 in abdominal pain (p = 0.0167) and flatulence (p = 0.0373). We also detected a statistically significant increase in the quality of life of patients receiving the active treatment from baseline to visit 3 (p < 0.0001).
Conclusions: Treatment with oligo- and polysaccharides and reticulated protein is safe, improving IBS symptoms and quality of life of patients with diarrhoea-predominant IBS.
Keywords: Irritable bowel syndrome; abdominal pain; efficacy; flatulence; mucosal protectors; quality of life; reticulated protein; stools; vegetable oligo-saccharides; vegetable polysaccharides.
Figures


Similar articles
-
Symptomatic efficacy of beidellitic montmorillonite in irritable bowel syndrome: a randomized, controlled trial.Aliment Pharmacol Ther. 2005 Feb 15;21(4):435-44. doi: 10.1111/j.1365-2036.2005.02330.x. Aliment Pharmacol Ther. 2005. PMID: 15709995 Clinical Trial.
-
Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial.United European Gastroenterol J. 2019 Oct;7(8):1093-1101. doi: 10.1177/2050640619862721. Epub 2019 Jul 3. United European Gastroenterol J. 2019. PMID: 31662866 Free PMC article. Clinical Trial.
-
A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome.Gastroenterol Clin Biol. 2008 Feb;32(2):147-52. doi: 10.1016/j.gcb.2007.06.001. Epub 2008 Mar 4. Gastroenterol Clin Biol. 2008. PMID: 18387426 Clinical Trial.
-
Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study.Gastroenterology. 2013 Aug;145(2):329-38.e1. doi: 10.1053/j.gastro.2013.04.006. Epub 2013 Apr 9. Gastroenterology. 2013. PMID: 23583433 Clinical Trial.
-
Update on the Management of Diarrhea-Predominant Irritable Bowel Syndrome: Focus on Rifaximin and Eluxadoline.Pharmacotherapy. 2016 Mar;36(3):300-16. doi: 10.1002/phar.1712. Epub 2016 Mar 11. Pharmacotherapy. 2016. PMID: 26971716 Review.
Cited by
-
Long-term safety and efficacy study of a medical device containing xyloglucan, pea protein reticulated with tannins and xylo-oligosaccharides, in patients with diarrhoea-predominant irritable bowel syndrome.Therap Adv Gastroenterol. 2021 May 30;14:17562848211020570. doi: 10.1177/17562848211020570. eCollection 2021. Therap Adv Gastroenterol. 2021. PMID: 34104216 Free PMC article.
-
Present and Future Therapeutic Approaches to Barrier Dysfunction.Front Nutr. 2021 Oct 28;8:718093. doi: 10.3389/fnut.2021.718093. eCollection 2021. Front Nutr. 2021. PMID: 34778332 Free PMC article. Review.
-
Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections.Int J Mol Sci. 2018 Jun 22;19(7):1844. doi: 10.3390/ijms19071844. Int J Mol Sci. 2018. PMID: 29932149 Free PMC article.
-
Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: a multicentre, randomised, double-blind, parallel group, clinical study.Transl Gastroenterol Hepatol. 2018 Sep 25;3:72. doi: 10.21037/tgh.2018.09.11. eCollection 2018. Transl Gastroenterol Hepatol. 2018. PMID: 30511026 Free PMC article.
-
The gut microbiome and irritable bowel syndrome.F1000Res. 2018 Jul 9;7:F1000 Faculty Rev-1029. doi: 10.12688/f1000research.14592.1. eCollection 2018. F1000Res. 2018. PMID: 30026921 Free PMC article. Review.
References
-
- Whelan K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc Nutr Soc 2013; 72: 288–298. - PubMed
-
- Minocha A, Johnson WD, Abell TL, et al. Prevalence, sociodemography, and quality of life of older versus younger patients with irritable bowel syndrome: A population-based study. Dig Dis Sci 2006; 51: 446–453. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

