Neuronal Trans-Differentiation in Prostate Cancer Cells
- PMID: 27403603
- PMCID: PMC5815867
- DOI: 10.1002/pros.23221
Neuronal Trans-Differentiation in Prostate Cancer Cells
Abstract
Background: Neuroendocrine (NE) differentiation in prostate cancer (PCa) is an aggressive phenotype associated with therapy resistance. The complete phenotype of these cells is poorly understood. Clinical classification is based predominantly on the expression of standard NE markers.
Methods: We analyzed the phenotype of NE carcinoma of the prostate utilizing in vitro methods, in silico, and immunohistochemical analyses of human disease.
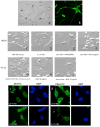
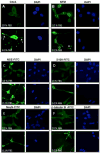
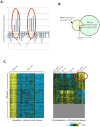
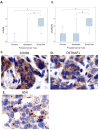
Results: LNCaP cells, subjected to a variety of stressors (0.1% [v/v] fetal bovine serum, cyclic AMP) induced a reproducible phenotype consistent with neuronal trans-differentiation. Cells developed long cytoplasmic processes resembling neurons. As expected, serum deprived cells had decreased expression in androgen receptor and prostate specific antigen. A significant increase in neuronal markers also was observed. Gene array analysis demonstrated that LNCaP cells subjected to low serum or cAMP showed statistically significant manifestation of a human brain gene expression signature. In an in silico experiment using human data, we identified that only hormone resistant metastatic prostate cancer showed enrichment of the "brain profile." Gene ontology analysis demonstrated categories involved in neuronal differentiation. Three neuronal markers were validated in a large human tissue cohort.
Conclusion: This study proposes that the later stages of PCa evolution involves neuronal trans-differentiation, which would enable PCa cells to acquire independence from the neural axis, critical in primary tumors. Prostate 76:1312-1325, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: nerves; neuroendocrine; neuronal trans-differentiation; neurons; prostate cancer; resistance phenotype; small cell.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
References
-
- Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79(2):235–46. Epub 1997/02/01. - PubMed
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Mattelaer J, Lopez Torecilla J, Pfeffer JR, Lino Cutajar C, Zurlo A, Pierart M. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–6. Epub 2002/07/20. - PubMed
-
- Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/nejm199707313370502. Epub 1997/07/31. - DOI - PubMed
-
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–7. doi: 10.1001/jama.292.7.821. Epub 2004/08/19. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

