Catalytic Carbocation Generation Enabled by the Mesolytic Cleavage of Alkoxyamine Radical Cations
- PMID: 27403637
- PMCID: PMC5102159
- DOI: 10.1002/anie.201604619
Catalytic Carbocation Generation Enabled by the Mesolytic Cleavage of Alkoxyamine Radical Cations
Abstract
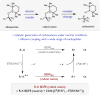
A new catalytic method is described to access carbocation intermediates via the mesolytic cleavage of alkoxyamine radical cations. In this process, electron transfer between an excited state oxidant and a TEMPO-derived alkoxyamine substrate gives rise to a radical cation with a remarkably weak C-O bond. Spontaneous scission results in the formation of the stable nitroxyl radical TEMPO(.) as well as a reactive carbocation intermediate that can be intercepted by a wide range of nucleophiles. Notably, this process occurs under neutral conditions and at comparatively mild potentials, enabling catalytic cation generation in the presence of both acid sensitive and easily oxidized nucleophilic partners.
Keywords: alkoxyamines; carbocations; mesolytic cleavage; photoredox catalysis; radical cations.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Olah GA, Prakash GKS. Carbocation Chemistry. Wiley; Hoboken, NJ: 2004.
- Naredle RR, Klumpp DA. Chem Rev. 2013;113:6905–6948. - PubMed
-
- Mori K, Sueoka S, Akiyama T. J Am Chem Soc. 2011;133:2424–2426. - PubMed
- Li H, Li W, Liu W, He Z, Li Z. Angew Chem Int Ed. 2011;50:2975–2978. - PubMed
- Angew Chem. 2011;123:3031–3034.
- Lv J, Zhang Q, Zhong X, Luo S. J Am Chem Soc. 2015;137:15576–15583. - PubMed
- Zhang F, Das S, Walkinshaw AJ, Casitas A, Taylor M, Suero MG, Gaunt MJ. J Am Chem Soc. 2014;136:8851–8854. - PubMed
- Nitsch D, Huber SM, PÖthig A, Narayanan A, Olah GA, Prakash GKS, Bach T. J Am Chem Soc. 2014;136:2851–2857. - PubMed
- Rueping M, Uria U, Lin MY, Atodiresei I. J Am Chem Soc. 2011;133:3732–3735. - PubMed
-
-
For a remarkable recent example, see: Loach RP, Fenton OS, Movassaghi M. J Am Chem Soc. 2016;138:1057–1064.
-
-
- Brak K, Jacobsen EN. Angew Chem Int Ed. 2013;52:534–561. - PMC - PubMed
- Angew Chem. 2013;125:558–588.
- Mahlau M, List B. Angew Chem Int Ed. 2013;52:518–533. - PubMed
- Angew Chem. 2013;125:540–556.
- Phipps RJ, Hamilton GL, Toste FD. Nat Chem. 2012;4:603–614. - PubMed
- Mittal N, Lippert KM, Kanta De C, Klauber EG, Emge TJ, Schreiner PR, Seidel D. J Am Chem Soc. 2015;137:5748–5758. - PubMed
-
- McNaught AD, Wilkinson A. IUPAC Compendium of Chemical Terminology, 2nd ed (the “Gold Book”) Blackwell Scientific Publications, Oxford; 1997.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources