Biochemical and proteomic characterization of retrovirus Gag based microparticles carrying melanoma antigens
- PMID: 27403717
- PMCID: PMC4941533
- DOI: 10.1038/srep29425
Biochemical and proteomic characterization of retrovirus Gag based microparticles carrying melanoma antigens
Abstract
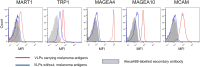
Extracellular vesicles are membraneous particles released by a variety of cells into the extracellular microenvironment. Retroviruses utilize the cellular vesiculation pathway for virus budding/assembly and the retrovirus Gag protein induces the spontaneous formation of microvesicles or virus-like particles (VLPs) when expressed in the mammalian cells. In this study, five different melanoma antigens, MAGEA4, MAGEA10, MART1, TRP1 and MCAM, were incorporated into the VLPs and their localization within the particles was determined. Our data show that the MAGEA4 and MAGEA10 proteins as well as MCAM are expressed on the surface of VLPs. The compartmentalization of exogenously expressed cancer antigens within the VLPs did not depend on the localization of the protein within the cell. Comparison of the protein content of VLPs by LC-MS/MS-based label-free quantitative proteomics showed that VLPs carrying different cancer antigens are very similar to each other, but differ to some extent from VLPs without recombinant antigen. We suggest that retrovirus Gag based virus-like particles carrying recombinant antigens have a potential to be used in cancer immunotherapy.
Figures




Similar articles
-
Type 1 diabetes pathogenesis is modulated by spontaneous autoimmune responses to endogenous retrovirus antigens in NOD mice.Eur J Immunol. 2017 Mar;47(3):575-584. doi: 10.1002/eji.201646755. Epub 2017 Feb 6. Eur J Immunol. 2017. PMID: 28083937
-
Characterization of influenza H1N1 Gag virus-like particles and extracellular vesicles co-produced in HEK-293SF.Vaccine. 2019 Nov 8;37(47):7100-7107. doi: 10.1016/j.vaccine.2019.07.057. Epub 2019 Jul 26. Vaccine. 2019. PMID: 31358407
-
Differential budding efficiencies of human T-cell leukemia virus type I (HTLV-I) Gag and Gag-Pro polyproteins from insect and mammalian cells.Virology. 2000 Dec 20;278(2):597-609. doi: 10.1006/viro.2000.0663. Virology. 2000. PMID: 11118382
-
Distinct Particle Morphologies Revealed through Comparative Parallel Analyses of Retrovirus-Like Particles.J Virol. 2016 Aug 26;90(18):8074-84. doi: 10.1128/JVI.00666-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27356903 Free PMC article.
-
MAGE-A family: attractive targets for cancer immunotherapy.Vaccine. 2011 Nov 3;29(47):8496-500. doi: 10.1016/j.vaccine.2011.09.014. Epub 2011 Sep 18. Vaccine. 2011. PMID: 21933694 Review.
Cited by
-
How the Intrinsically Disordered N-Terminus of Cancer/Testis Antigen MAGEA10 Is Responsible for Its Expression, Nuclear Localisation and Aberrant Migration.Biomolecules. 2023 Nov 24;13(12):1704. doi: 10.3390/biom13121704. Biomolecules. 2023. PMID: 38136576 Free PMC article.
-
Production of Oral Vaccines Based on Virus-Like Particles Pseudotyped with Protozoan-Surface Proteins.Methods Mol Biol. 2022;2410:503-537. doi: 10.1007/978-1-0716-1884-4_26. Methods Mol Biol. 2022. PMID: 34914065
-
Synthetic biology for bioengineering virus-like particle vaccines.Biotechnol Bioeng. 2019 Apr;116(4):919-935. doi: 10.1002/bit.26890. Epub 2018 Dec 31. Biotechnol Bioeng. 2019. PMID: 30597533 Free PMC article. Review.
-
Antibody response against cancer-testis antigens MAGEA4 and MAGEA10 in patients with melanoma.Oncol Lett. 2018 Jul;16(1):211-218. doi: 10.3892/ol.2018.8684. Epub 2018 May 10. Oncol Lett. 2018. PMID: 29928403 Free PMC article.
-
Selective Isolation of Retroviruses from Extracellular Vesicles by Intact Virion Immunoprecipitation.Bio Protoc. 2018 Sep 5;8(17):e3005. doi: 10.21769/BioProtoc.3005. eCollection 2018 Sep 5. Bio Protoc. 2018. PMID: 34395797 Free PMC article.
References
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 13, 269–288 (1967). - PubMed
-
- Théry C., Ostrowski M. & Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 9, 581–593 (2009). - PubMed
-
- van Dommelen S. et al.. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 161, 635–644 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

