Health Technology Assessment of pathogen reduction technologies applied to plasma for clinical use
- PMID: 27403740
- PMCID: PMC4942318
- DOI: 10.2450/2016.0065-16
Health Technology Assessment of pathogen reduction technologies applied to plasma for clinical use
Abstract
Although existing clinical evidence shows that the transfusion of blood components is becoming increasingly safe, the risk of transmission of known and unknown pathogens, new pathogens or re-emerging pathogens still persists. Pathogen reduction technologies may offer a new approach to increase blood safety. The study is the output of collaboration between the Italian National Blood Centre and the Post-Graduate School of Health Economics and Management, Catholic University of the Sacred Heart, Rome, Italy. A large, multidisciplinary team was created and divided into six groups, each of which addressed one or more HTA domains.Plasma treated with amotosalen + UV light, riboflavin + UV light, methylene blue or a solvent/detergent process was compared to fresh-frozen plasma with regards to current use, technical features, effectiveness, safety, economic and organisational impact, and ethical, social and legal implications. The available evidence is not sufficient to state which of the techniques compared is superior in terms of efficacy, safety and cost-effectiveness. Evidence on efficacy is only available for the solvent/detergent method, which proved to be non-inferior to untreated fresh-frozen plasma in the treatment of a wide range of congenital and acquired bleeding disorders. With regards to safety, the solvent/detergent technique apparently has the most favourable risk-benefit profile. Further research is needed to provide a comprehensive overview of the cost-effectiveness profile of the different pathogen-reduction techniques. The wide heterogeneity of results and the lack of comparative evidence are reasons why more comparative studies need to be performed.
Figures

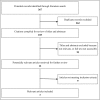
Similar articles
-
Update on pathogen reduction technology for therapeutic plasma: an overview.Transfus Apher Sci. 2006 Aug;35(1):83-90. doi: 10.1016/j.transci.2006.02.004. Epub 2006 Aug 24. Transfus Apher Sci. 2006. PMID: 16934528 Review.
-
Solvent/detergent-treated plasma: a tale of 30 years of experience.Expert Rev Hematol. 2015 Jun;8(3):367-74. doi: 10.1586/17474086.2015.1016906. Epub 2015 Feb 19. Expert Rev Hematol. 2015. PMID: 25695198 Review.
-
Component pathogen inactivation: a critical review.Vox Sang. 2013 Apr;104(3):183-99. doi: 10.1111/j.1423-0410.2012.01662.x. Epub 2012 Nov 8. Vox Sang. 2013. PMID: 23134556 Review.
-
Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology.Blood Transfus. 2017 Jul;15(4):357-364. doi: 10.2450/2017.0320-16. Epub 2017 May 11. Blood Transfus. 2017. PMID: 28665269 Free PMC article. Review.
-
Pathogen reduction of blood components.Transfus Apher Sci. 2008 Aug;39(1):75-82. doi: 10.1016/j.transci.2008.05.003. Epub 2008 Jul 3. Transfus Apher Sci. 2008. PMID: 18602343 Review.
Cited by
-
The Preclinical Validation of 405 nm Light Parasiticidal Efficacy on Leishmania donovani in Ex Vivo Platelets in a Rag2-/- Mouse Model.Microorganisms. 2024 Jan 29;12(2):280. doi: 10.3390/microorganisms12020280. Microorganisms. 2024. PMID: 38399684 Free PMC article.
-
Intrathecal Immunoselective Nanopheresis for Alzheimer's Disease: What and How? Why and When?Int J Mol Sci. 2024 Oct 2;25(19):10632. doi: 10.3390/ijms251910632. Int J Mol Sci. 2024. PMID: 39408961 Free PMC article. Review.
-
Initial Results of a Prospective Study and Identification of New Strategies to Increase Traceability of Plasma-derived Medicines.Iran J Pharm Res. 2018 Winter;17(Suppl):145-150. Iran J Pharm Res. 2018. PMID: 29796039 Free PMC article.
-
The Rise of SARS-CoV-2 Variants and the Role of Convalescent Plasma Therapy for Management of Infections.Life (Basel). 2021 Jul 23;11(8):734. doi: 10.3390/life11080734. Life (Basel). 2021. PMID: 34440478 Free PMC article. Review.
-
Transfusion Transmissible Infections in Blood Donors in the Province of Bié, Angola, during a 15-Year Follow-Up, Imply the Need for Pathogen Reduction Technologies.Pathogens. 2021 Dec 17;10(12):1633. doi: 10.3390/pathogens10121633. Pathogens. 2021. PMID: 34959588 Free PMC article.
References
-
- Benjamin RJ, McLaughlin LS. Plasma components: properties, differences, and uses. Transfusion. 2012;52:9S–19S. - PubMed
-
- Sherwood L. Human Physiology: From Cells to Systems. 8th ed. Belmont, CA: Brooks/Cole-Cengage Learning; 2013.
-
- Strumia MM, McGraw JJ. The development of plasma preparations for transfusions. Ann Intern Med. 1941;15:80–8.
-
- Gosselin RC, Marshall C, Dwyre DM, et al. Coagulation profile of liquid-state plasma. Transfusion. 2013;53:579–90. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
