Rosette-Disrupting Effect of an Anti-Plasmodial Compound for the Potential Treatment of Plasmodium falciparum Malaria Complications
- PMID: 27403804
- PMCID: PMC4941523
- DOI: 10.1038/srep29317
Rosette-Disrupting Effect of an Anti-Plasmodial Compound for the Potential Treatment of Plasmodium falciparum Malaria Complications
Abstract
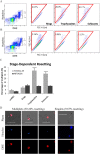
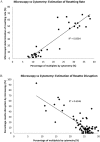
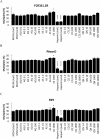
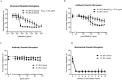
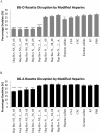
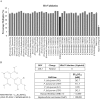
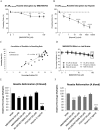
The spread of artemisinin-resistant parasites could lead to higher incidence of patients with malaria complications. However, there are no current treatments that directly dislodge sequestered parasites from the microvasculature. We show that four common antiplasmodial drugs do not disperse rosettes (erythrocyte clusters formed by malaria parasites) and therefore develop a cell-based high-throughput assay to identify potential rosette-disrupting compounds. A pilot screen of 2693 compounds identified Malaria Box compound MMV006764 as a potential candidate. Although it reduced rosetting by a modest 20%, MMV006764 was validated to be similarly effective against both blood group O and A rosettes of three laboratory parasite lines. Coupled with its antiplasmodial activity and drug-likeness, MMV006764 represents the first small-molecule compound that disrupts rosetting and could potentially be used in a resource-limited setting to treat patients deteriorating rapidly from malaria complications. Such dual-action drugs that simultaneously restore microcirculation and reduce parasite load could significantly reduce malaria morbidity and mortality.
Conflict of interest statement
M.W. is a co-founder and board member of Dilaforette, a company developing drugs for severe malaria. All other authors declare no financial conflicts of interest.
Figures
Similar articles
-
Plasmodium falciparum rosetting protects schizonts against artemisinin.EBioMedicine. 2021 Nov;73:103680. doi: 10.1016/j.ebiom.2021.103680. Epub 2021 Nov 5. EBioMedicine. 2021. PMID: 34749300 Free PMC article.
-
Erythrocyte rosetting in Plasmodium falciparum malaria--with special reference to the pathogenesis of cerebral malaria.Scand J Infect Dis Suppl. 1993;86:1-79. Scand J Infect Dis Suppl. 1993. PMID: 8493454
-
Identification via a Parallel Hit Progression Strategy of Improved Small Molecule Inhibitors of the Malaria Purine Uptake Transporter that Inhibit Plasmodium falciparum Parasite Proliferation.ACS Infect Dis. 2019 Oct 11;5(10):1738-1753. doi: 10.1021/acsinfecdis.9b00168. Epub 2019 Aug 14. ACS Infect Dis. 2019. PMID: 31373203 Free PMC article.
-
How genomics is contributing to the fight against artemisinin-resistant malaria parasites.Acta Trop. 2015 Aug;148:1-7. doi: 10.1016/j.actatropica.2015.04.007. Epub 2015 Apr 21. Acta Trop. 2015. PMID: 25910626 Review.
-
Molecular mechanisms and biological importance of Plasmodium falciparum erythrocyte rosetting.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:323-9. doi: 10.1590/s0074-02761992000700054. Mem Inst Oswaldo Cruz. 1992. PMID: 1285315 Review.
Cited by
-
The Medicines for Malaria Venture Malaria Box contains inhibitors of protein secretion in Plasmodium falciparum blood stage parasites.Traffic. 2022 Sep;23(9):442-461. doi: 10.1111/tra.12862. Epub 2022 Aug 15. Traffic. 2022. PMID: 36040075 Free PMC article.
-
Malaria Parasite Plasmodium falciparum Proteins on the Surface of Infected Erythrocytes as Targets for Novel Drug Discovery.Biochemistry (Mosc). 2022 Jan;87(Suppl 1):S192-S177. doi: 10.1134/S0006297922140152. Biochemistry (Mosc). 2022. PMID: 35501996 Free PMC article. Review.
-
Frequent GU wobble pairings reduce translation efficiency in Plasmodium falciparum.Sci Rep. 2017 Apr 7;7(1):723. doi: 10.1038/s41598-017-00801-9. Sci Rep. 2017. PMID: 28389662 Free PMC article.
-
Red blood cell mannoses as phagocytic ligands mediating both sickle cell anaemia and malaria resistance.Nat Commun. 2021 Mar 19;12(1):1792. doi: 10.1038/s41467-021-21814-z. Nat Commun. 2021. PMID: 33741926 Free PMC article.
-
Antibodies in children with malaria to PfEMP1, RIFIN and SURFIN expressed at the Plasmodium falciparum parasitized red blood cell surface.Sci Rep. 2018 Feb 19;8(1):3262. doi: 10.1038/s41598-018-21026-4. Sci Rep. 2018. PMID: 29459776 Free PMC article.
References
-
- Kaul D. K., Roth E. F. Jr., Nagel R. L., Howard R. J. & Handunnetti S. M. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78, 812–819 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

