Conjugation of Methotrexate-Amino Derivatives to Macromolecules through Carboxylate Moieties Is Superior Over Conventional Linkage to Amino Residues: Chemical, Cell-Free and In Vitro Characterizations
- PMID: 27403959
- PMCID: PMC4942054
- DOI: 10.1371/journal.pone.0158352
Conjugation of Methotrexate-Amino Derivatives to Macromolecules through Carboxylate Moieties Is Superior Over Conventional Linkage to Amino Residues: Chemical, Cell-Free and In Vitro Characterizations
Abstract
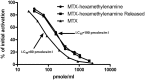
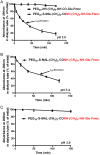
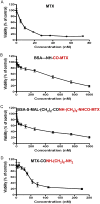
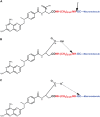
In this study, we examined the possibility of introducing methotrexate (MTX) to the carboxylate rather than to the ε-amino side chains of proteins. We found that MTX-amino compounds covalently linked to the carboxylate moieties of macromolecules, undergo unusual peptide-bond cleavage, with the release of the MTX amino derivatives from the conjugates. This event takes place at an accelerated rate under acidic conditions, and at a slower rate at physiological pH values. The glutamate portion of MTX is responsible for this behavior, with little or no contribution of the p-aminobenzoate-pteridine ring that is linked to the α-amino side chain of the glutamate. Carboxylate-linked Fmoc-Glu-γ-CONH-(CH2)6-NH2 undergoes hydrolysis in a nearly indistinguishable fashion. A free α carboxylate moiety is essential for this effect. Carboxylate linked Fmoc-glutamic-amide-γ-CONH-(CH2)6-NH2 undergoes no hydrolysis under acidic conditions. Based on these findings, we engineered a cysteine specific MTX containing reagent. Its linkage to bovine serum albumin (BSA) yielded a conjugate with profound antiproliferative efficacy in a MTX-sensitive glioma cell line. In conclusion, carboxylate linked MTX-amino derivatives in particular, and carboxylate linked R-α-GLU-γ amino compounds in general are equipped with'built-in chemical machinery' that releases them under mild acidic conditions.
Conflict of interest statement
Figures




Similar articles
-
Growth inhibition, drug load, and degradation studies of gelatin/methotrexate conjugates.Int J Pharm. 2006 Feb 3;308(1-2):90-9. doi: 10.1016/j.ijpharm.2005.10.037. Epub 2005 Dec 19. Int J Pharm. 2006. PMID: 16361072
-
Synthesis and properties of a biodegradable polymer-drug conjugate: Methotrexate-poly(glycerol adipate).Colloids Surf B Biointerfaces. 2018 Jul 1;167:115-125. doi: 10.1016/j.colsurfb.2018.03.048. Epub 2018 Mar 28. Colloids Surf B Biointerfaces. 2018. PMID: 29631222
-
Gadolinium texaphyrin-methotrexate conjugates. Towards improved cancer chemotherapeutic agents.Org Biomol Chem. 2005 Sep 21;3(18):3290-6. doi: 10.1039/b503664j. Epub 2005 Aug 4. Org Biomol Chem. 2005. PMID: 16132091
-
TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation.Int J Mol Sci. 2016 Jun 18;17(6):967. doi: 10.3390/ijms17060967. Int J Mol Sci. 2016. PMID: 27322261 Free PMC article.
-
Chemotherapeutic potential of methotrexate peptides.Adv Enzyme Regul. 1988;27:3-13. doi: 10.1016/0065-2571(88)90005-2. Adv Enzyme Regul. 1988. PMID: 3074629 Review.
Cited by
-
Albumin-EDTA-Vanadium Is a Powerful Anti-Proliferative Agent, Following Entrance into Glioma Cells via Caveolae-Mediated Endocytosis.Pharmaceutics. 2021 Sep 25;13(10):1557. doi: 10.3390/pharmaceutics13101557. Pharmaceutics. 2021. PMID: 34683850 Free PMC article.
-
Albumin-Methotrexate Prodrug Analogues That Undergo Intracellular Reactivation Following Entrance into Cancerous Glioma Cells.Pharmaceutics. 2021 Dec 28;14(1):71. doi: 10.3390/pharmaceutics14010071. Pharmaceutics. 2021. PMID: 35056966 Free PMC article.
References
-
- Shechter Y, Mironchik M, Saul A, Gershonov E, Precido-Patt L, Sasson K, et al. New Technologies to Prolong Life-time of Peptide and Protein Drugs In vivo. International Journal of Peptide Research and Therapeutics. 2007;13:105–17. 10.1007/s10989-006-9052-1 - DOI
-
- Shechter Y, Sasson K, Marcus Y, Rubinraut S, Lev-Goldman V, Fridkin M. Establishing the principle of reversibility in peptide/protein and small-molecule therapy. Ther Deliv. 2012;3(1):17–23. Epub 2012/07/27. . - PubMed
-
- Gershonov E, Shechter Y, Fridkin M. New concept for long-acting insulin: spontaneous conversion of an inactive modified insulin to the active hormone in circulation: 9-fluorenylmethoxycarbonyl derivative of insulin. Diabetes. 1999;48(7):1437–42. Epub 1999/07/02. . - PubMed
-
- Gershonov E, Goldwaser I, Fridkin M, Shechter Y. A novel approach for a water-soluble long-acting insulin prodrug: design, preparation, and analysis of [(2-sulfo)-9-fluorenylmethoxycarbonyl](3)-insulin. J Med Chem. 2000;43(13):2530–7. Epub 2000/07/13. jm990533m [pii]. . - PubMed
-
- Shechter Y, Preciado-Patt L, Schreiber G, Fridkin M. Prolonging the half-life of human interferon-alpha 2 in circulation: Design, preparation, and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7- interferon-alpha 2. Proc Natl Acad Sci U S A. 2001;98(3):1212–7. Epub 2001/02/07. 10.1073/pnas.98.3.1212 98/3/1212 [pii]. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

