AplusB: A Web Application for Investigating A + B Designs for Phase I Cancer Clinical Trials
- PMID: 27403961
- PMCID: PMC4942070
- DOI: 10.1371/journal.pone.0159026
AplusB: A Web Application for Investigating A + B Designs for Phase I Cancer Clinical Trials
Abstract
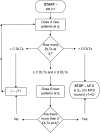
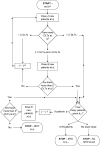
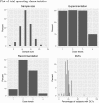
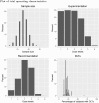
In phase I cancer clinical trials, the maximum tolerated dose of a new drug is often found by a dose-escalation method known as the A + B design. We have developed an interactive web application, AplusB, which computes and returns exact operating characteristics of A + B trial designs. The application has a graphical user interface (GUI), requires no programming knowledge and is free to access and use on any device that can open an internet browser. A customised report is available for download for each design that contains tabulated operating characteristics and informative plots, which can then be compared with other dose-escalation methods. We present a step-by-step guide on how to use this application and provide several illustrative examples of its capabilities.
Conflict of interest statement
Figures



References
-
- National Cancer Institute. Dictionary of Cancer Terms; 2016. Available from: http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=572160.
-
- Babb JS, Rogatko A. Bayesian methods for phase I cancer clinical trials In: Geller NL, editor. Advances in Clinical Trial Biostatistics. New York, NY: Marcel Dekker; 2004. p. 1–40.
-
- NCI. Common Terminology Criteria for Adverse Events v4.0; 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

