Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis
- PMID: 27404358
- PMCID: PMC4942852
- DOI: 10.1016/j.devcel.2016.06.009
Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis
Abstract
Basement membranes (BMs) are planar protein networks that support epithelial function. Regulated changes to BM architecture can also contribute to tissue morphogenesis, but how epithelia dynamically remodel their BMs is unknown. In Drosophila, elongation of the initially spherical egg chamber correlates with the generation of a polarized network of fibrils in its surrounding BM. Here, we use live imaging and genetic manipulations to determine how these fibrils form. BM fibrils are assembled from newly synthesized proteins in the pericellular spaces between the egg chamber's epithelial cells and undergo oriented insertion into the BM by directed epithelial migration. We find that a Rab10-based secretion pathway promotes pericellular BM protein accumulation and fibril formation. Finally, by manipulating this pathway, we show that BM fibrillar structure influences egg chamber morphogenesis. This work highlights how regulated protein secretion can synergize with tissue movement to build a polarized BM architecture that controls tissue shape.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



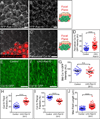

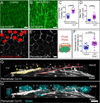
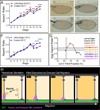
Comment in
-
Tissue Sculpting by Fibrils.Dev Cell. 2016 Jul 11;38(1):1-3. doi: 10.1016/j.devcel.2016.06.028. Dev Cell. 2016. PMID: 27404350
References
-
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. - PubMed
-
- Aurich F, Dahmann C. A Mutation in fat2 Uncouples Tissue Elongation from Global Tissue Rotation. Cell Rep. 2016;14:2503–2510. - PubMed
-
- Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 2001;11:1317–1327. - PubMed
-
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, Wilhelm JE, Murphy TD, Levis RW, Matunis E, Srivali N, Hoskins RA, Spradling AC. The Carnegie Protein Trap Library: A Versatile Tool for Drosophila Developmental Studies. Genetics. 2007;175:1505–1531. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

