Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation
- PMID: 27404668
- PMCID: PMC5459484
- DOI: 10.1002/cncr.30180
Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation
Abstract
Background: High-dose, post-transplantation cyclophosphamide (PTCy) to prevent graft-versus-host disease (GVHD) has improved outcomes in haploidentical (HAPLO) stem cell transplantation (SCT). However, it remains unclear whether this strategy is effective in SCT from 1-antigen human leukocyte antigen (HLA)-mismatched unrelated donors (9/10 MUD) and how the outcomes of these patients compare with those of haploidentical transplantation recipients.
Methods: A parallel, 2-arm, nonrandomized phase 2 clinical trial was conducted of melphalan-based reduced-intensity conditioning with PTCy, tacrolimus, and mycophenolate mofetil to prevent GVHD in patients with high-risk hematologic malignancies who underwent HAPLO (n = 60) or 9/10 MUD (n = 46) SCT.
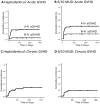
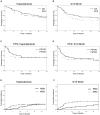
Results: The 1-year overall and progression-free survival rates were 70% and 60%, respectively, in the HAPLO arm and 60% and 47%, respectively, in the 9/10 MUD arm. The day +100 cumulative incidence of grade II to IV acute GVHD and grade III to IV acute GVHD was 28% and 3%, respectively, in the HAPLO arm and 33% and 13%, respectively, in the 9/10 MUD arm. The 2-year cumulative incidence of chronic GVHD was 24% in the HAPLO arm and 19% in the 9/10 MUD arm. The 1-year cumulative incidence of nonrelapse mortality was 21% in the HAPLO arm and 31% in the 9/10 MUD arm, and the 1-year relapse rate was 19% in the HAPLO arm and 25% in the 9/10 MUD arm.
Conclusions: Although this was a nonrandomized study and could not serve as a direct comparison between the 2 groups, the authors conclude that PTCy-based GVHD prophylaxis is effective for both HAPLO and 9/10 MUD SCTs. Prospective randomized trials will be required to compare the efficacies of alternative donor options for patients lacking HLA-matched donors. Cancer 2016;122:3316-3326. © 2016 American Cancer Society.
Keywords: 9/10 matched unrelated donors; graft-versus-host disease; haploidentical transplantation; hematologic malignancies; post-transplantation cyclophosphamide.
© 2016 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
Dean Lee reports licensing fees from Ziopharm Oncology and Intrexon Corporation; personal fees from Ziopharm Oncology, Courier Therapeutics, Intellia Therapeutics, Shire, and Sanofi Oncology; and holds ownership and is a board member of Therapeutics, all outside the submitted work. Qaiser Bashir reports research funding from Celgene and Takeda and serves on the Takeda and Spectrum Pharmaceuticals advisory boards, all outside the submitted work. Chitra Hosing reports a grant for clinical trials from Celgene, Inc; and personal fees from Sanofi, Aviara Pharmaceuticals, Seattle Genetics, and Cardinal Health, all outside the submitted work.
Figures

References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
-
- Allan DS, Takach S, Smith S, Goldman M. Impact of declining fertility rates in Canada on donor options in blood and marrow transplantation. Biol Blood Marrow Transplant. 2009;15:1634–1637. - PubMed
-
- Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematology Am Soc Hematol Educ Program. 2008:412–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

