Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility
- PMID: 27404793
- PMCID: PMC5164965
- DOI: 10.1016/j.jphysparis.2016.07.001
Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility
Abstract
The basal forebrain cholinergic projection system to the cortex mediates essential aspects of visual attention performance, including the detection of cues and the response to performance challenges (top-down control of attention). Higher levels of top-down control are mediated via elevated levels of cholinergic neuromodulation. The neuronal choline transporter (CHT) strongly influences the synthesis and release of acetylcholine (ACh). As the capacity of the CHT to import choline into the neuron is a major, presynaptic determinant of cholinergic neuromodulation, we hypothesize that genetically-imposed CHT capacity variation impacts the balance of bottom-up versus top-down control of visual attention. Following a brief review of the cognitive concepts relevant for this hypothesis, we describe the key results from our research in mice and humans that possess genetically-imposed changes in choline uptake capacity. CHT subcapacity is associated with poor top-down attentional control and attenuated (cholinergic) activation of right frontal regions. Conversely, mice overexpressing the CHT, and humans expressing a CHT variant hypothesized to enhance choline transporter function, are relatively resistant to challenges of visual attention performance. Genetic or environmental modulation of CHT expression and function may be associated with vulnerabilities for cognitive disorders.
Keywords: Acetylcholine; Attention; Choline transporter; Genetics; Humans; Rodents.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


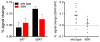
References
-
- Bazalakova MH, Blakely RD. The high-affinity choline transporter: a critical protein for sustaining cholinergic signaling as revealed in studies of genetically altered mice. Handb Exp Pharmacol. 2006;(175):525–544. - PubMed
-
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6(5):411–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

