Antifibrotic Activity of Human Placental Amnion Membrane-Derived CD34+ Mesenchymal Stem/Progenitor Cell Transplantation in Mice With Thioacetamide-Induced Liver Injury
- PMID: 27405780
- PMCID: PMC5070504
- DOI: 10.5966/sctm.2015-0343
Antifibrotic Activity of Human Placental Amnion Membrane-Derived CD34+ Mesenchymal Stem/Progenitor Cell Transplantation in Mice With Thioacetamide-Induced Liver Injury
Abstract
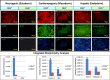
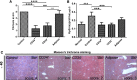
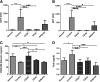
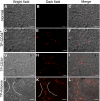
: Liver fibrosis represents the end stage of chronic liver inflammatory diseases and is defined by the abnormal accumulation of extracellular matrix in the liver. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension. Liver transplantation has been the most effective treatment for these diseases, but the procedure is limited by the shortage of suitable donors. Mesenchymal stromal cells (MSCs) have shown great potential in the treatment of chronic inflammatory diseases associated with fibrosis. This study aimed to evaluate the therapeutic effect of MSC-based cell transplantation as an alternative treatment for liver fibrosis. A CD34-positive subpopulation of human placental amnion membrane-derived stem/progenitor cells (CD34+ AMSPCs) was isolated through the depletion of CD34-negative stromal fibroblasts (CD34- AMSFCs) facilitated by CD34 fluorescence-activated cell sorting, enriched and expanded ex vivo. These cells express pluripotency markers and demonstrate multidirectional differentiation potentials. Comparative analysis was made between CD34+ AMSPCs and CD34- AMSFCs in terms of the expressions of stemness surface markers, embryonic surface antigens, and multilineage differentiation potentials. A mouse model of liver fibrosis was established by thioacetamide (TAA) administration. When injected into the spleen of TAA-injured mice, human placental amnion membrane-derived MSCs (hAM-MSCs) can engraft into the injury site, ameliorate liver fibrosis, and restore liver function, as shown by pathological and blood biochemical analysis and downregulated gene expressions associated with liver damage. CD34+ AMSPCs represent a more primitive subset of hAM-MSCs and could be a suitable candidate with a potentially better safety profile for cell-based therapy in treatment of liver diseases associated with fibrosis.
Significance: In this study, a CD34+ subpopulation of stem/progenitor cells derived from neonatal placental amnion membrane, denoted as CD34+ AMSPCs, were identified, enriched, and characterized. These cells are highly proliferative, express mesenchymal stromal cells and pluripotent stem cell markers, and demonstrate multidirectional differentiation potentials, indicating their promising application in clinical regenerative therapies. CD34+ AMSPC transplantation ameliorated liver fibrosis in mice with drug-induced liver injury. These cells represent a potential therapeutic agent for treating liver diseases associated with fibrosis.
Keywords: Antifibrotic; Human amnion membrane-derived CD34+ MSC; Thioacetamide-induced liver fibrosis; Transplantation.
©AlphaMed Press.
Figures


Similar articles
-
Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury.J Cell Physiol. 2018 Dec;233(12):9330-9344. doi: 10.1002/jcp.26413. Epub 2018 Jun 19. J Cell Physiol. 2018. PMID: 29266258
-
Ex vivo expansion of hematopoietic stem- and progenitor cells from cord blood in coculture with mesenchymal stroma cells from amnion, chorion, Wharton's jelly, amniotic fluid, cord blood, and bone marrow.Tissue Eng Part A. 2013 Dec;19(23-24):2577-85. doi: 10.1089/ten.tea.2013.0073. Tissue Eng Part A. 2013. PMID: 24308543
-
Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model.BMC Gastroenterol. 2014 Nov 25;14:198. doi: 10.1186/s12876-014-0198-6. BMC Gastroenterol. 2014. PMID: 25425284 Free PMC article.
-
Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis.Stem Cells. 2014 Nov;32(11):2818-23. doi: 10.1002/stem.1818. Stem Cells. 2014. PMID: 25154380 Review.
-
Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases?Transplantation. 2016 Dec;100(12):2548-2557. doi: 10.1097/TP.0000000000001426. Transplantation. 2016. PMID: 27495745 Review.
Cited by
-
The Role of B Cells in PE Pathophysiology: A Potential Target for Perinatal Cell-Based Therapy?Int J Mol Sci. 2021 Mar 26;22(7):3405. doi: 10.3390/ijms22073405. Int J Mol Sci. 2021. PMID: 33810280 Free PMC article. Review.
-
Extraembryonic Mesenchymal Stromal/Stem Cells in Liver Diseases: A Critical Revision of Promising Advanced Therapy Medicinal Products.Cells. 2022 Mar 23;11(7):1074. doi: 10.3390/cells11071074. Cells. 2022. PMID: 35406638 Free PMC article. Review.
-
CM from intact hAM: an easily obtained product with relevant implications for translation in regenerative medicine.Stem Cell Res Ther. 2021 Oct 12;12(1):540. doi: 10.1186/s13287-021-02607-z. Stem Cell Res Ther. 2021. PMID: 34641958 Free PMC article.
-
Recent Development in Therapeutic Cardiac Patches.Front Cardiovasc Med. 2020 Nov 27;7:610364. doi: 10.3389/fcvm.2020.610364. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33330673 Free PMC article. Review.
-
B Lymphocytes as Targets of the Immunomodulatory Properties of Human Amniotic Mesenchymal Stromal Cells.Front Immunol. 2020 Jun 9;11:1156. doi: 10.3389/fimmu.2020.01156. eCollection 2020. Front Immunol. 2020. PMID: 32582218 Free PMC article.
References
-
- Crawford JM. Pathologic basis of disease: The liver and biliary tract. Philadelphia, PA: Saunders; 1999. pp. 845–891.
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. - PubMed
-
- Chernykh ER, Starostina NM, Paltsev AI, et al. Autologous bone marrow cells in the treatment of cirrhosis of the liver. Bull Exp Biol Med. 2007;144:640–645. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

