Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma
- PMID: 27405814
- PMCID: PMC4941022
- DOI: 10.1186/s12885-016-2512-x
Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma
Abstract
Background: Stereotactic body radiation therapy (SBRT) has been an emerging non-invasive treatment modality for patients with hepatocellular carcinoma (HCC) when curative treatments cannot be applied. In this study, we report our clinical experience with Cyberknife SBRT for unresectable HCC and evaluate the efficacy and clinical outcomes of this highly sophisticated treatment technology.
Methods: Between 2008 and 2012, 115 patients with unresectable HCC treated with Cyberknife SBRT were retrospectively analyzed. Doses ranged from 26 Gy to 40 Gy were given in 3 to 5 fractions for 3 to 5 consecutive days. The cumulative probability of survival was calculated according to the Kaplan-Meier method and compared using log-rank test. Univariate and multivariate analysis were performed using Cox proportional hazard models.
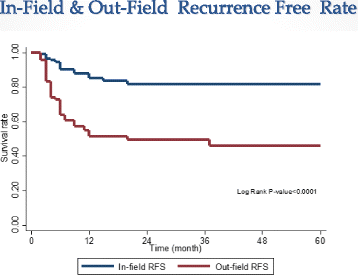
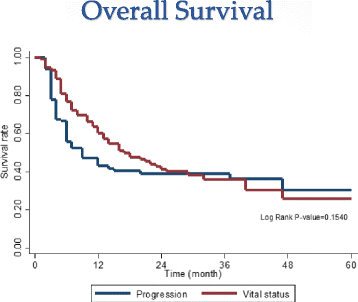
Results: The median follow-up was 15.5 months (range, 2-60 months). Based on Response Evaluation and Criteria in Solid Tumors (RECIST). We found that 48.7 % of patients achieved a complete response and 40 % achieved a partial response. Median survival was 15 months (4-25 months). Overall survival (OS) at 1- and 2-years was 63.5 %(54-71.5 %) and 41.3 % (31.6-50.6 %), respectively, while 1- and 2- years Progression-free Survival (PFS) rates were 42.8 %(33.0-52.2 %) and 38.8 % (29.0-48.4 %). Median progression was 6 months (3-16 months). In-field recurrence free survival at 1 and 2 years was 85.3 % (76.2-91.1 %) and 81.6 % (72.2-88.6 %), respectively, while the 1- and 2-years out-field recurrence free survival were 52.5 % (41.2-60.8 %) and 49.5 %(38.9-59.2 %), respectively. Multivariate analysis revealed that Child-Pugh score (A vs. B), Portal vein tumor thrombosis (positive vs. negative), Tumor size (≤4 cm vs >4-9 cm /≥10 cm), and tumor response after SBRT (CR vs. PR/stable) were independent predictors of OS. Acute toxicity was mostly transient and tolerable.
Conclusions: Cyberknife SBRT appears to be an effective non-invasive treatment for local unresectable HCC with low risk of severe toxicity. These results suggested that Cyberknife SBRT can be a good alternative treatment for unresectable HCC unsuitable for standard treatment.
Keywords: Cyberknife; Hepatocellular carcinoma; Stereotactic body radiation therapy.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

