BMP and retinoic acid regulate anterior-posterior patterning of the non-axial mesoderm across the dorsal-ventral axis
- PMID: 27406002
- PMCID: PMC4947171
- DOI: 10.1038/ncomms12197
BMP and retinoic acid regulate anterior-posterior patterning of the non-axial mesoderm across the dorsal-ventral axis
Abstract
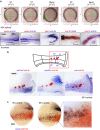
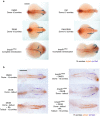
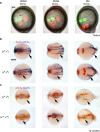
Despite the fundamental importance of patterning along the dorsal-ventral (DV) and anterior-posterior (AP) axes during embryogenesis, uncertainty exists in the orientation of these axes for the mesoderm. Here we examine the origin and formation of the zebrafish kidney, a ventrolateral mesoderm derivative, and show that AP patterning of the non-axial mesoderm occurs across the classic gastrula stage DV axis while DV patterning aligns along the animal-vegetal pole. We find that BMP signalling acts early to establish broad anterior and posterior territories in the non-axial mesoderm while retinoic acid (RA) functions later, but also across the classic DV axis. Our data support a model in which RA on the dorsal side of the embryo induces anterior kidney fates while posterior kidney progenitors are protected ventrally by the RA-catabolizing enzyme Cyp26a1. This work clarifies our understanding of vertebrate axis orientation and establishes a new paradigm for how the kidney and other mesodermal derivatives arise during embryogenesis.
Figures






References
-
- Lane M. C. & Sheets M. D. Rethinking axial patterning in amphibians. Dev. Dyn. 225, 434–447 (2002). - PubMed
-
- Kumano G. & Smith W. C. Revisions to the Xenopus gastrula fate map: implications for mesoderm induction and patterning. Dev. Dyn. 225, 409–421 (2002). - PubMed
-
- Warga R. M. & Nusslein-Volhard C. Origin and development of the zebrafish endoderm. Development 126, 827–838 (1999). - PubMed
-
- Fauny J. D., Thisse B. & Thisse C. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of Nodal to BMP activity. Development 136, 3811–3819 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

