Beta-amyloid 1-42 monomers, but not oligomers, produce PHF-like conformation of Tau protein
- PMID: 27406053
- PMCID: PMC5013016
- DOI: 10.1111/acel.12500
Beta-amyloid 1-42 monomers, but not oligomers, produce PHF-like conformation of Tau protein
Abstract
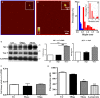
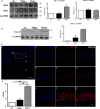
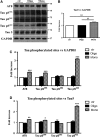
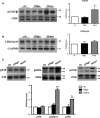
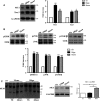
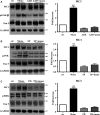
The mechanistic relationship between amyloid β1-42 (Aβ1-42) and the alteration of Tau protein are debated. We investigated the effect of Aβ1-42 monomers and oligomers on Tau, using mice expressing wild-type human Tau that do not spontaneously develop Tau pathology. After intraventricular injection of Aβ1-42, mice were sacrificed after 3 h or 4 days. The short-lasting treatment with Aβ monomers, but not oligomers, showed a conformational PHF-like change of Tau, together with hyperphosphorylation. The same treatment induced increase in concentration of GSK3 and MAP kinases. The inhibition of the kinases rescued the Tau changes. Aβ monomers increased the levels of total Tau, through the inhibition of proteasomal degradation. Aβ oligomers reproduced all the aforementioned alterations only after 4 days of treatment. It is known that Aβ1-42 monomers foster synaptic activity. Our results suggest that Aβ monomers physiologically favor Tau activity and dendritic sprouting, whereas their excess causes Tau pathology. Moreover, our study indicates that anti-Aβ therapies should be targeted to Aβ1-42 monomers too.
Keywords: Alzheimer's disease; MAPK; PHF; beta-amyloid; hTau mice; tau protein.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures
References
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86, 582–590. - PubMed
-
- Beeg M, Stravalaci M, Bastone A, Salmona M, Gobbi M (2011) A modified protocol to prepare seed‐free starting solutions of amyloid‐β (Aβ)₁₋₄₀ and Aβ₁₋₄₂ from the corresponding depsipeptides. Anal. Biochem. 411, 297–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

