Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea
- PMID: 27406165
- PMCID: PMC5036235
- DOI: 10.1136/thoraxjnl-2016-208501
Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea
Abstract
Background: Non-invasive ventilation (NIV) is an effective form of treatment in patients with obesity hypoventilation syndrome (OHS) who have concomitant severe obstructive sleep apnoea (OSA). However, there is a paucity of evidence on the efficacy of NIV in patients with OHS without severe OSA. We performed a multicentre randomised clinical trial to determine the comparative efficacy of NIV versus lifestyle modification (control group) using daytime arterial carbon dioxide tension (PaCO2) as the main outcome measure.
Methods: Between May 2009 and December 2014 we sequentially screened patients with OHS without severe OSA. Participants were randomised to NIV versus lifestyle modification and were followed for 2 months. Arterial blood gas parameters, clinical symptoms, health-related quality of life assessments, polysomnography, spirometry, 6-min walk distance test, blood pressure measurements and healthcare resource utilisation were evaluated. Statistical analysis was performed using intention-to-treat analysis.
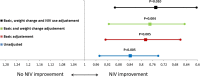
Results: A total of 365 patients were screened of whom 58 were excluded. Severe OSA was present in 221 and the remaining 86 patients without severe OSA were randomised. NIV led to a significantly larger improvement in PaCO2 of -6 (95% CI -7.7 to -4.2) mm Hg versus -2.8 (95% CI -4.3 to -1.3) mm Hg, (p<0.001) and serum bicarbonate of -3.4 (95% CI -4.5 to -2.3) versus -1 (95% CI -1.7 to -0.2 95% CI) mmol/L (p<0.001). PaCO2 change adjusted for NIV compliance did not further improve the inter-group statistical significance. Sleepiness, some health-related quality of life assessments and polysomnographic parameters improved significantly more with NIV than with lifestyle modification. Additionally, there was a tendency towards lower healthcare resource utilisation in the NIV group.
Conclusions: NIV is more effective than lifestyle modification in improving daytime PaCO2, sleepiness and polysomnographic parameters. Long-term prospective studies are necessary to determine whether NIV reduces healthcare resource utilisation, cardiovascular events and mortality.
Trial registration number: NCT01405976; results.
Keywords: Non invasive ventilation; Sleep apnoea.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


Comment in
-
NIV for OHS without severe OSAS: is it worth it?Thorax. 2016 Oct;71(10):877-8. doi: 10.1136/thoraxjnl-2016-208986. Epub 2016 Aug 9. Thorax. 2016. PMID: 27507900 No abstract available.
References
-
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
