Asymmetrical seeding of MSCs into fibrin-poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis
- PMID: 27406210
- PMCID: PMC6093257
- DOI: 10.1002/term.2194
Asymmetrical seeding of MSCs into fibrin-poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis
Abstract
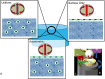
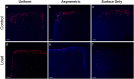
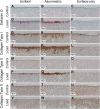
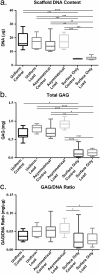
Mesenchymal stem cells (MSCs) are currently being investigated as candidate cells for regenerative medicine approaches for the repair of damaged articular cartilage. For these cells to be used clinically, it is important to understand how they will react to the complex loading environment of a joint in vivo. In addition to investigating alternative cell sources, it is also important for the structure of tissue-engineered constructs and the organization of cells within them to be developed and, if possible, improved. A custom built bioreactor was used to expose human MSCs to a combination of shear and compression loading. The MSCs were either evenly distributed throughout fibrin-poly(ester-urethane) scaffolds or asymmetrically seeded with a small proportion seeded on the surface of the scaffold. The effect of cell distribution on the production and deposition of cartilage-like matrix in response to mechanical load mimicking in vivo joint loading was then investigated. The results show that asymmetrically seeding the scaffold led to markedly improved tissue development based on histologically detectable matrix deposition. Consideration of cell location, therefore, is an important aspect in the development of regenerative medicine approaches for cartilage repair. This is particularly relevant when considering the natural biomechanical environment of the joint in vivo and patient rehabilitation protocols. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: bioreactor; cartilage repair; mesenchymal stem cell; multi-axial load; poly-ester-urethane; shear.
Copyright © 2016 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures

References
-
- Atala A, Bauer SB, Soker S et al 2006; Tissue‐engineered autologous bladders for patients needing cystoplasty. Lancet 367: 1241–1246. - PubMed
-
- Benya PD, Shaffer JD. 1982; Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30: 215–224. - PubMed
-
- Boissard I, Bourban PE, Tami AE et al 2009; Nanohydroxyapatite/poly(ester urethane) scaffold for bone tissue engineering. Acta Biomater 5: 3316–3327. - PubMed
-
- Brittberg M, Lindahl A, Nilsson A et al 1994; Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331: 889–895. - PubMed
-
- Cherubino P, Grassi FA, Bulgheroni P, et al 2003; Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong) 11: 10–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

