Development of a Full-Thickness Human Gingiva Equivalent Constructed from Immortalized Keratinocytes and Fibroblasts
- PMID: 27406216
- PMCID: PMC4991602
- DOI: 10.1089/ten.TEC.2016.0066
Development of a Full-Thickness Human Gingiva Equivalent Constructed from Immortalized Keratinocytes and Fibroblasts
Abstract
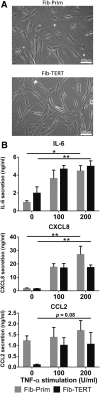
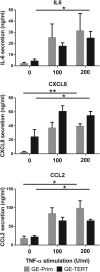
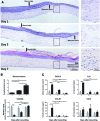
Organotypic models make it possible to investigate the unique properties of oral mucosa in vitro. For gingiva, the use of human primary keratinocytes (KC) and fibroblasts (Fib) is limited due to the availability and size of donor biopsies. The use of physiologically relevant immortalized cell lines would solve these problems. The aim of this study was to develop fully differentiated human gingiva equivalents (GE) constructed entirely from cell lines, to compare them with the primary cell counterpart (Prim), and to test relevance in an in vitro wound healing assay. Reconstructed gingiva epithelium on a gingiva fibroblast-populated collagen hydrogel was constructed from cell lines (keratinocytes: TERT or HPV immortalized; fibroblasts: TERT immortalized) and compared to GE-Prim and native gingiva. GE were characterized by immunohistochemical staining for proliferation (Ki67), epithelial differentiation (K10, K13), and basement membrane (collagen type IV and laminin 5). To test functionality of GE-TERT, full-thickness wounds were introduced. Reepithelialization, fibroblast repopulation of hydrogel, metabolic activity (MTT assay), and (pro-)inflammatory cytokine release (enzyme-linked immunosorbent assay) were assessed during wound closure over 7 days. Significant differences in basal KC cytokine secretion (IL-1α, IL-18, and CXCL8) were only observed between KC-Prim and KC-HPV. When Fib-Prim and Fib-TERT were stimulated with TNF-α, no differences were observed regarding cytokine secretion (IL-6, CXCL8, and CCL2). GE-TERT histology, keratin, and basement membrane protein expression very closely represented native gingiva and GE-Prim. In contrast, the epithelium of GE made with HPV-immortalized KC was disorganized, showing suprabasal proliferating cells, limited keratinocyte differentiation, and the absence of basement membrane proteins. When a wound was introduced into the more physiologically relevant GE-TERT model, an immediate inflammatory response (IL-6, CCL2, and CXCL8) was observed followed by complete reepithelialization. Seven days after wounding, tissue integrity, metabolic activity, and cytokine levels had returned to the prewounded state. In conclusion, immortalized human gingiva KC and fibroblasts can be used to make physiologically relevant GE, which resemble either the healthy gingiva or a neoplastic disease model. These organotypic models will provide valuable tools to investigate oral mucosa biology and can also be used as an animal alternative for drug targeting, vaccination studies, microbial biofilm studies, and testing new therapeutics.
Figures


References
-
- Presland R.B., and Dale B.A. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med 11, 383, 2000 - PubMed
-
- Vriens A.P., et al. . Comparison of autologous full-thickness gingiva and skin substitutes for wound healing. Cell Transplant 17, 1199, 2008 - PubMed
-
- Gibbs S., and Ponec M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch Oral Biol 45, 149, 2000 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
