Mortality Reduction in Septic Shock by Plasma Adsorption (ROMPA): a protocol for a randomised clinical trial
- PMID: 27406647
- PMCID: PMC4947802
- DOI: 10.1136/bmjopen-2016-011856
Mortality Reduction in Septic Shock by Plasma Adsorption (ROMPA): a protocol for a randomised clinical trial
Abstract
Introduction: There is a lack of evidence in the efficacy of the coupled plasma filtration adsorption (CPFA) to reduce the mortality rate in septic shock. To fill this gap, we have designed the ROMPA study (Mortality Reduction in Septic Shock by Plasma Adsorption) to confirm whether treatment with an adequate dose of treated plasma by CPFA could confer a clinical benefit.
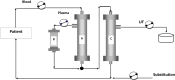
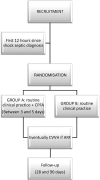
Methods and analysis: Our study is a multicentric randomised clinical trial with a 28-day and 90-day follow-up and allocation ratio 1:1. Its aim is to clarify whether the application of high doses of CPFA (treated plasma ≥0.20 L/kg/day) in the first 3 days after randomisation, in addition to the current clinical practice, is able to reduce hospital mortality in patients with septic shock in intensive care units (ICUs) at 28 and 90 days after initiation of the therapy. The study will be performed in 10 ICUs in the Southeast of Spain which follow the same protocol in this disease (based on the Surviving Sepsis Campaign). Our trial is designed to be able to demonstrate an absolute mortality reduction of 20% (α=0.05; 1-β=0.8; n=190(95×2)). The severity of the process, ensuring the recruitment of patients with a high probability of death (50% in the control group), will be achieved through an adequate stratification by using both severity scores and classical definitions of severe sepsis/septic shock and dynamic parameters. Our centres are fully aware of the many pitfalls associated with previous medical device trials. Trying to reduce these problems, we have developed a training programme to improve the CPFA use (especially clotting problems).
Ethics and dissemination: The protocol was approved by the Ethics Committees of all the participant centres. The findings of the trial will be disseminated through peer-reviewed journals, as well as national and international conference presentations.
Trial registration number: NCT02357433; Pre-results.
Keywords: INFECTIOUS DISEASES; STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
