A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A
- PMID: 27406695
- PMCID: PMC5059953
- DOI: 10.1111/irv.12408
A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A
Abstract
Background: Serum antibody to influenza can be used to identify past exposure and measure current immune status. The two most common methods for measuring this are the hemagglutination inhibition assay (HI) and the viral neutralization assay (NT), which have not been systematically compared for a large number of influenza viruses.
Methods: A total of 151 study participants from near Guangzhou, China, were enrolled in 2009 and provided serum. HI and NT assays were performed for 12 historic and recently circulating strains of seasonal influenza A. We compared titers using Spearman correlation and fit models to predict NT using HI results.
Results: We observed high positive mean correlation between HI and NT assays (Spearman's rank correlation, ρ=.86) across all strains. Correlation was highest within subtypes and within close proximity in time. Overall, an HI=20 corresponded to NT=10, and HI=40 corresponded to NT=20. Linear regression of log(NT) on log(HI) was statistically significant, with age modifying this relationship. Strain-specific area under a curve (AUC) indicated good accuracy (>80%) for predicting NT with HI.
Conclusions: While we found high overall correspondence of titers between NT and HI assays for seasonal influenza A, no exact equivalence between assays could be determined. This was further complicated by correspondence between titers changing with age. These findings support generalized comparison of results between assays and give further support for use of the hemagglutination inhibition assay over the more resource intensive viral neutralization assay for seasonal influenza A, although attention should be given to the effect of age on these assays.
Keywords: cross-protection; hemagglutination inhibition test; immunity; influenza; microneutralization test; neutralization test.
© 2016 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures
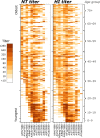
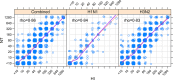


References
-
- Hayden F, Palese P. Influenza virus In: Richman DD, Whitley RJ, Hayden FG, ed. Clinical Virology. New York: ASM Press; 2009:943–976.
-
- Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine. 2007;25:4056–4063. - PubMed
-
- Wood JM, Major D, Heath A, et al. Reproducibility of serology assays for pandemic influenza H1N1: collaborative study to evaluate a candidate WHO International Standard. Vaccine. 2012;30:210–217. - PubMed
-
- Centers for Disease Control and Prevention . Antibodies Cross‐Reactive to Influenza A (H3N2) Variant Virus and Impact of 2010–11 Seasonal Influenza Vaccine on Cross‐Reactive Antibodies — United States. Atlanta, GA: Centers for Disease Control and Prevention; 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

