Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients
- PMID: 27406733
- PMCID: PMC4942815
- DOI: 10.1038/srep29506
Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients
Abstract
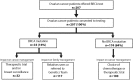
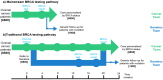
Advances in DNA sequencing have made genetic testing fast and affordable, but limitations of testing processes are impeding realisation of patient benefits. Ovarian cancer exemplifies the potential value of genetic testing and the shortcomings of current pathways to access testing. Approximately 15% of ovarian cancer patients have a germline BRCA1 or BRCA2 mutation which has substantial implications for their personal management and that of their relatives. Unfortunately, in most countries, routine implementation of BRCA testing for ovarian cancer patients has been inconsistent and largely unsuccessful. We developed a rapid, robust, mainstream genetic testing pathway in which testing is undertaken by the trained cancer team with cascade testing to relatives performed by the genetics team. 207 women with ovarian cancer were offered testing through the mainstream pathway. All accepted. 33 (16%) had a BRCA mutation. The result informed management of 79% (121/154) women with active disease. Patient and clinician feedback was very positive. The pathway offers a 4-fold reduction in time and 13-fold reduction in resource requirement compared to the conventional testing pathway. The mainstream genetic testing pathway we present is effective, efficient and patient-centred. It can deliver rapid, robust, large-scale, cost-effective genetic testing of BRCA1 and BRCA2 and may serve as an exemplar for other genes and other diseases.
Figures

Comment in
-
Streamlined genetic testing pathway is developed for women with ovarian cancer.BMJ. 2016 Jul 13;354:i3900. doi: 10.1136/bmj.i3900. BMJ. 2016. PMID: 27418655 No abstract available.
References
-
- Ferlay J. et al.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 136, E359–386 (2015). - PubMed
-
- Pal T. et al.. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 104, 2807–2816 (2005). - PubMed
-
- Risch H. A. et al.. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute 98, 1694–1706 (2006). - PubMed
-
- Schrader K. A. et al.. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstetrics and Gynecology 120, 235–240 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

