Overexpression of an Arabidopsis cysteine-rich receptor-like protein kinase, CRK5, enhances abscisic acid sensitivity and confers drought tolerance
- PMID: 27406784
- PMCID: PMC5014153
- DOI: 10.1093/jxb/erw266
Overexpression of an Arabidopsis cysteine-rich receptor-like protein kinase, CRK5, enhances abscisic acid sensitivity and confers drought tolerance
Abstract
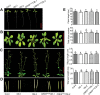
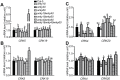
Receptor-like kinases (RLKs) have been reported to regulate many developmental and defense process, but only a few members have been functionally characterized. In the present study, our observations suggest that one of the RLKs, a membrane-localized cysteine-rich receptor-like protein kinase, CRK5, is involved in abscisic acid (ABA) signaling in Arabidopsis thaliana Overexpression of CRK5 increases ABA sensitivity in ABA-induced early seedling growth arrest and promotion of stomatal closure and inhibition of stomatal opening. Interestingly, and importantly, overexpression of CRK5 enhances plant drought tolerance without affecting plant growth at the mature stages and plant productivity. Transgenic lines overexpressing a mutated form of CRK5, CRK5 (K372E) with the change of the 372nd conserved amino acid residue from lysine to glutamic acid in its kinase domain, result in wild-type ABA and drought responses, supporting the role of CRK5 in ABA signaling. The loss-of-function mutation of the CRK5 gene does not affect the ABA response, while overexpression of two homologs of CRK5, CRK4 and CRK19, confers ABA responses, suggesting that these CRK members function redundantly. We further showed that WRKY18, WRKY40 and WRKY60 transcription factors repress the expression of CRK5, and that CRK5 likely functions upstream of ABI2 in ABA signaling. These findings help in understanding the complex ABA signaling network.
Keywords: ABI2; CRK5; WRKY18; WRKY40; WRKY60.; abscisic acid; drought tolerance; receptor-like kinase.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures










References
-
- Acharya BR, Raina S, Maqbool SB, Jagadeeswaran G, Mosher SL, Appel HM, Schultz JC, Klessig DF, Raina R. 2007. Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. The Plant Journal 50, 488–499. - PubMed
-
- Bai L, Zhang GZ, Zhou Y, Zhang Z, Wang W, Du YY, Wu ZY, Song CP. 2009. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana . The Plant Journal 60, 314–327. - PubMed
-
- Chen KG, Du LQ, Chen ZX. 2003. Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis . Plant Molecular Biology 53, 61–74. - PubMed
-
- Chen KG, Fan B, Du L, Chen ZX. 2004. Activation of hypersensitive cell death by pathogen-induced receptor-like protein kinases from Arabidopsis . Plant Molecular Biology 56, 271–283. - PubMed
-
- Deng KQ, Wang QM, Zeng JX, Guo XH, Zhao XY, Tang DY, Liu XM. 2009. A lectin receptor kinase positively regulates aba response during seed germination and is involved in salt and osmotic stress response. Journal of Plant Biology 52, 493–500.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

