Endocrine tumors associated with the vagus nerve
- PMID: 27406876
- PMCID: PMC5022786
- DOI: 10.1530/ERC-16-0241
Endocrine tumors associated with the vagus nerve
Abstract
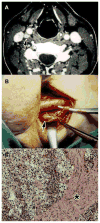
The vagus nerve (cranial nerve X) is the main nerve of the parasympathetic division of the autonomic nervous system. Vagal paragangliomas (VPGLs) are a prime example of an endocrine tumor associated with the vagus nerve. This rare, neural crest tumor constitutes the second most common site of hereditary head and neck paragangliomas (HNPGLs), most often in relation to mutations in the succinate dehydrogenase complex subunit D (SDHD) gene. The treatment paradigm for VPGL has progressively shifted from surgery to abstention or therapeutic radiation with curative-like outcomes. Parathyroid tissue and parathyroid adenoma can also be found in close association with the vagus nerve in intra or paravagal situations. Vagal parathyroid adenoma can be identified with preoperative imaging or suspected intraoperatively by experienced surgeons. Vagal parathyroid adenomas located in the neck or superior mediastinum can be removed via initial cervicotomy, while those located in the aortopulmonary window require a thoracic approach. This review particularly emphasizes the embryology, molecular genetics, and modern imaging of these tumors.
Keywords: diagnostic imaging; hyperparathyroidism; paragangliomas; vagus nerve.
© 2016 Society for Endocrinology.
Conflict of interest statement
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

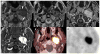
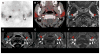
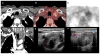
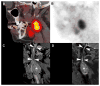

References
-
- al Zahrani A, Levine MA. Primary hyperparathyroidism. Lancet. 1997;349:1233–1238. - PubMed
-
- Archier A, Varoquaux A, Garrigue P, Montava M, Guerin C, Gabriel S, Beschmout E, Morange I, Fakhry N, Castinetti F, et al. Prospective comparison of (68)Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43:1248–1257. - PubMed
-
- Arnault V, Beaulieu A, Lifante JC, Sitges Serra A, Sebag F, Mathonnet M, Hamy A, Meurisse M, Carnaille B, Kraimps JL. Multicenter study of 19 aortopulmonary window parathyroid tumors: the challenge of embryologic origin. World journal of surgery. 2010;34:2211–2216. - PubMed
-
- Arnold SM, Strecker R, Scheffler K, Spreer J, Schipper J, Neumann HP, Klisch J. Dynamic contrast enhancement of paragangliomas of the head and neck: evaluation with time-resolved 2D MR projection angiography. European radiology. 2003;13:1608–1611. - PubMed
-
- Baker CVH. The Embryology of Vagal Sensory Neurons. In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Boca Raton: CRC Press; 2005. pp. 3–26.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

