Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma
- PMID: 27407058
- PMCID: PMC5129499
- DOI: 10.1002/hed.24522
Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma
Abstract
Background: Cutaneous head and neck melanoma has poor outcomes and limited treatment options. In OPTiM, a phase 3 study in patients with unresectable stage IIIB/IIIC/IV melanoma, intralesional administration of the oncolytic virus talimogene laherparepvec improved durable response rate (DRR; continuous response ≥6 months) compared with subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF).
Methods: Retrospective review of OPTiM identified patients with cutaneous head and neck melanoma given talimogene laherparepvec (n = 61) or GM-CSF (n = 26). Outcomes were compared between talimogene laherparepvec and GM-CSF treated patients with cutaneous head and neck melanoma.
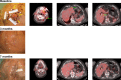
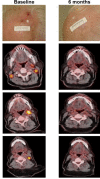
Results: DRR was higher for talimogene laherparepvec-treated patients than for GM-CSF treated patients (36.1% vs 3.8%; p = .001). A total of 29.5% of patients had a complete response with talimogene laherparepvec versus 0% with GM-CSF. Among talimogene laherparepvec-treated patients with a response, the probability of still being in response after 12 months was 73%. Median overall survival (OS) was 25.2 months for GM-CSF and had not been reached with talimogene laherparepvec.
Conclusion: Treatment with talimogene laherparepvec was associated with improved response and survival compared with GM-CSF in patients with cutaneous head and neck melanoma. © 2016 Wiley Periodicals, Inc. Head Neck 38: 1752-1758, 2016.
Keywords: cancer immunotherapy; cutaneous head and neck melanoma; oncolytic virus; talimogene laherparepvec.
© 2016 The Authors Head & Neck Published by Wiley Periodicals, Inc.
Figures


References
-
- Fadaki N, Li R, Parrett B, et al. Is head and neck melanoma different from trunk and extremity melanomas with respect to sentinel lymph node status and clinical outcome? Ann Surg Oncol 2013;20:3089–3097. - PubMed
-
- Garbe C, Büttner P, Bertz J, et al. Primary cutaneous melanoma. Identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer 1995;75:2484–2491. - PubMed
-
- Golger A, Young DS, Ghazarian D, Neligan PC. Epidemiological features and prognostic factors of cutaneous head and neck melanoma: a population‐based study. Arch Otolaryngol Head Neck Surg 2007;133:442–447. - PubMed
-
- Kienstra MA, Padhya TA. Head and neck melanoma. Cancer Control 2005;12:242–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

