Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer's disease
- PMID: 27407064
- PMCID: PMC4942833
- DOI: 10.1038/srep29760
Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer's disease
Abstract
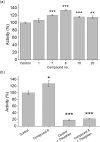
Neprilysin (NEP) is the most important Aβ-degrading enzyme. Its expression level decreases with age and inversely correlated with amyloid accumulation, suggesting its correlation with the late-onset of Alzheimer's disease. Recently, many reports showed that upregulating NEP level is a promising strategy in the prevention and therapy of Alzheimer's disease. Here, we used a sensitive fluorescence-based Aβ digestion assay to screen 25 curcumin analogs for their ability to upregulate NEP activity. To our surprise, four compounds, dihydroxylated curcumin, monohydroxylated demethoxycurcumin, and mono- and di-hydroxylated bisdemethoxycurcumin, increased NEP activity, while curcumin did not. The ability of these polyhydroxycurcuminoids to upregulate NEP was further confirmed by mRNA and protein expression levels in the cell and mouse models. Finally, feeding monohydroxylated demethoxycurcumin (also named demethylcurcumin) or dihydroxylated bisdemethoxycurcumin (also named bisdemethylcurcumin) to APPswe/PS1dE9 double transgenic mice upregulated NEP levels in the brain and reduced Aβ accumulation in the hippocampus and cortex. These polyhydroxycurcuminoids offer hope in the prevention of Alzheimer's disease.
Conflict of interest statement
There is potential competing financial interests. One US patent (US 9,074,238) has been granted.
Figures







References
-
- Iwata N., Higuchi M. & Saido T. C. Metabolism of amyloid-b peptide and Alzheimer’s disease. Pharmacol. Therapeut. 108, 129–148 (2005). - PubMed
-
- Wang D. S., Iwata N., Hama E., Saido T. C. & Dickson D. W. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 310, 236–241 (2003). - PubMed
-
- Hellstrom-Lindahl E., Ravid R. & Nordberg A. Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with A beta levels. Neurobiol. Aging 29, 210–221 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

