Recent developments on the role of epigenetics in obesity and metabolic disease
- PMID: 27408648
- PMCID: PMC4940755
- DOI: 10.1186/s13148-015-0101-5
Recent developments on the role of epigenetics in obesity and metabolic disease
Abstract
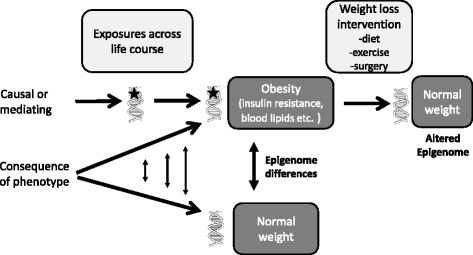
The increased prevalence of obesity and related comorbidities is a major public health problem. While genetic factors undoubtedly play a role in determining individual susceptibility to weight gain and obesity, the identified genetic variants only explain part of the variation. This has led to growing interest in understanding the potential role of epigenetics as a mediator of gene-environment interactions underlying the development of obesity and its associated comorbidities. Initial evidence in support of a role of epigenetics in obesity and type 2 diabetes mellitus (T2DM) was mainly provided by animal studies, which reported epigenetic changes in key metabolically important tissues following high-fat feeding and epigenetic differences between lean and obese animals and by human studies which showed epigenetic changes in obesity and T2DM candidate genes in obese/diabetic individuals. More recently, advances in epigenetic methodologies and the reduced cost of epigenome-wide association studies (EWAS) have led to a rapid expansion of studies in human populations. These studies have also reported epigenetic differences between obese/T2DM adults and healthy controls and epigenetic changes in association with nutritional, weight loss, and exercise interventions. There is also increasing evidence from both human and animal studies that the relationship between perinatal nutritional exposures and later risk of obesity and T2DM may be mediated by epigenetic changes in the offspring. The aim of this review is to summarize the most recent developments in this rapidly moving field, with a particular focus on human EWAS and studies investigating the impact of nutritional and lifestyle factors (both pre- and postnatal) on the epigenome and their relationship to metabolic health outcomes. The difficulties in distinguishing consequence from causality in these studies and the critical role of animal models for testing causal relationships and providing insight into underlying mechanisms are also addressed. In summary, the area of epigenetics and metabolic health has seen rapid developments in a short space of time. While the outcomes to date are promising, studies are ongoing, and the next decade promises to be a time of productive research into the complex interactions between the genome, epigenome, and environment as they relate to metabolic disease.
Keywords: DNA methylation; Developmental programming; Epigenetics; Obesity; Type 2 diabetes.
Figures

References
-
- WHO. WHO | Overweight and obesity. http://www.who.int/gho/ncd/risk_factors/overweight/en/index.html. Accessed 29 January 2015.
-
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

