Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms
- PMID: 27408701
- PMCID: PMC4926737
- DOI: 10.12688/f1000research.8206.1
Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms
Abstract
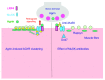
Myasthenia gravis is an autoimmune disease of the neuromuscular junction (NMJ) caused by antibodies that attack components of the postsynaptic membrane, impair neuromuscular transmission, and lead to weakness and fatigue of skeletal muscle. This can be generalised or localised to certain muscle groups, and involvement of the bulbar and respiratory muscles can be life threatening. The pathogenesis of myasthenia gravis depends upon the target and isotype of the autoantibodies. Most cases are caused by immunoglobulin (Ig)G1 and IgG3 antibodies to the acetylcholine receptor (AChR). They produce complement-mediated damage and increase the rate of AChR turnover, both mechanisms causing loss of AChR from the postsynaptic membrane. The thymus gland is involved in many patients, and there are experimental and genetic approaches to understand the failure of immune tolerance to the AChR. In a proportion of those patients without AChR antibodies, antibodies to muscle-specific kinase (MuSK), or related proteins such as agrin and low-density lipoprotein receptor-related protein 4 (LRP4), are present. MuSK antibodies are predominantly IgG4 and cause disassembly of the neuromuscular junction by disrupting the physiological function of MuSK in synapse maintenance and adaptation. Here we discuss how knowledge of neuromuscular junction structure and function has fed into understanding the mechanisms of AChR and MuSK antibodies. Myasthenia gravis remains a paradigm for autoantibody-mediated conditions and these observations show how much there is still to learn about synaptic function and pathological mechanisms.
Keywords: AChR; Myasthenia gravis; immunoglobulin; neuromuscular junction.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

