Treatment efficacy of (153)Sm-EDTMP for painful bone metastasis
- PMID: 27408839
- PMCID: PMC4937668
- DOI: 10.7508/aojnmb.2013.01.006
Treatment efficacy of (153)Sm-EDTMP for painful bone metastasis
Abstract
Introduction: Involvement of the skeleton can cause an excruciating pain in two-thirds of terminal patients with a history of malignancy. Due to several limitations of other therapies, such as analgesics, bisphosphonates, chemotherapy, hormonal therapy and external beam radiotherapy; bone-seeking radiopharmaceuticals have an important role in palliation of pain from bone metastases. Although these kinds of therapies have many advantages including the ability to treat multiple sites of tumoral involvement simultaneously, no significant confliction with other treatments, ease of administration and the potential to be used repetitively; in Iran using of this modality is not widely practiced. In this study we evaluated the clinical usefulness of Sm-153 lexidronamfor pain management of bone metastases.
Methods: 28 patients (14 males and 14 females) aged 38-77 years with a history of painful bone metastases caused by different cancers, not responding to conventional treatments were included in the study. All patients had a recent whole body bone scan indicating multiple bone metastases. 1 mCi/Kg Sm-153 lexidronam was injected intravenously to the patients. Whole body scintigraphy was done 3 or 18 hours post injection. Pain relief and quality of life have been evaluated by analog pain scale and Karnofsky index every week, respectively. Also, all patients were evaluated for hematological toxicity every two weeks. Active follow ups were performed.
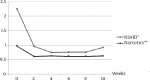
Results: 43% of patients showed the presence of the flare phenomenon during the first three days after Sm injection with a mean duration of 2.2 days. The pain relief began between 2 and 16 days post injection and the duration of pain palliation was in the range of 4 to 32 weeks (mean±SD=15.22±7.8). 64.3% of patients showed complete relief of pain and 21.4% achieved partial response to therapy. (Over all response to therapy was 85.7%). The lowest amount of peripheral blood cells was detected in the fourth week for RBCs and in the 6th week for WBCs and PLTs. No one experienced hematological toxicity induced problems.
Conclusion: Sm-153 lexidronam is an effective treatment for painful bone metastases. The complication rate is low and the quality of life of the patients after treatment would be significantly improved.
Keywords: 153Sm-EDTMP; Radionuclide Therapy; Samarium; painful bone metastasis.
Figures


Similar articles
-
Optimal timing of bisphosphonate administration in combination with samarium-153 oxabifore in the treatment of painful metastatic bone disease.World J Nucl Med. 2013 Jan;12(1):14-8. doi: 10.4103/1450-1147.113939. World J Nucl Med. 2013. PMID: 23961250 Free PMC article.
-
Our experience on pain palliation of bone metastasis with Sr-89 or Sm-153 in cancer patients resistant to a conventional analgesic therapy. A retrospective study.Clin Ter. 2009;160(3):193-9. Clin Ter. 2009. PMID: 19756320
-
Clinical Efficacy and Safety Comparison of 177Lu-EDTMP with 153Sm-EDTMP on an Equidose Basis in Patients with Painful Skeletal Metastases.J Nucl Med. 2015 Oct;56(10):1513-9. doi: 10.2967/jnumed.115.155762. Epub 2015 Aug 27. J Nucl Med. 2015. PMID: 26315829 Clinical Trial.
-
Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma.Expert Rev Anticancer Ther. 2007 Nov;7(11):1517-27. doi: 10.1586/14737140.7.11.1517. Expert Rev Anticancer Ther. 2007. PMID: 18020921 Review.
-
Radiopharmaceutical therapy for palliation of bone pain from osseous metastases.J Nucl Med. 2004 Aug;45(8):1358-65. J Nucl Med. 2004. PMID: 15299062 Review.
Cited by
-
The application of radionuclide therapy for breast cancer.Front Nucl Med. 2024 Jan 10;3:1323514. doi: 10.3389/fnume.2023.1323514. eCollection 2023. Front Nucl Med. 2024. PMID: 39355029 Free PMC article. Review.
-
Metastatic Bone Pain Palliation using (177)Lu-Ethylenediaminetetramethylene Phosphonic Acid.World J Nucl Med. 2015 May-Aug;14(2):109-15. doi: 10.4103/1450-1147.157124. World J Nucl Med. 2015. PMID: 26097421 Free PMC article.
-
Targeted Palliative Radionuclide Therapy for Metastatic Bone Pain.J Clin Med. 2020 Aug 12;9(8):2622. doi: 10.3390/jcm9082622. J Clin Med. 2020. PMID: 32806765 Free PMC article. Review.
-
Added value of hybrid SPECT with CT imaging for predicting poor therapeutic efficacy of 89Sr in patients with bone metastasis.Sci Rep. 2020 Dec 3;10(1):21207. doi: 10.1038/s41598-020-78372-5. Sci Rep. 2020. PMID: 33273651 Free PMC article.
-
Radiotheranostics - Precision Medicine in Nuclear Medicine and Molecular Imaging.Nanotheranostics. 2022 Jan 1;6(1):103-117. doi: 10.7150/ntno.64141. eCollection 2022. Nanotheranostics. 2022. PMID: 34976584 Free PMC article. Review.
References
-
- Saarto T, Janes R, Tenhunen M, Kouri M. Palliative radiotherapy in the treatment of skeletal metastases. Eur J Pain. 2002;6(5):323–30. - PubMed
-
- Mhiri A, Hassad R, Sellem A, Bahri H, Diakite R, Slim I, et al. [Treatment of painful bony metastases with Samarium 153-EDTMP in prostate carcinoma] Tunis Med. 2007 Jul;85(7):580–5. - PubMed
-
- Bauman G, Charette M, Reid R, Sathya J. Radiopharmaceuticals for the palliation of painful bone metastasis-a systemic review. Radiother Oncol. 2005 Jun;75(3):258–70. - PubMed
-
- Roque IFM, Martinez-Zapata MJ, Scott-Brown M, Alonso-Coello P. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev. 2011;7:CD003347. - PubMed
-
- Baczyk M. Radioisotope therapy of bone metastases. Nucl Med Rev Cent East Eur. 2011;14(2):96–104. - PubMed
LinkOut - more resources
Full Text Sources
