Production, biodistribution assessment and dosimetric evaluation of (177)Lu-TTHMP as an agent for bone pain palliation
- PMID: 27408879
- PMCID: PMC4937688
Production, biodistribution assessment and dosimetric evaluation of (177)Lu-TTHMP as an agent for bone pain palliation
Abstract
Objectives: Recently, bone-avid radiopharmaceuticals have been shown to have potential benefits for the treatment of widespread bone metastases. Although (177)Lu-triethylene tetramine hexa methylene phosphonic acid (abbreviated as (177)Lu-TTHMP), as an agent for bone pain palliation, has been evaluated in previous studies, there are large discrepancies between the obtained results. In this study, production, quality control, biodistribution, and dose evaluation of (177)Lu-TTHMP have been investigated and compared with the previously reported data.
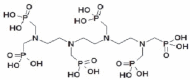
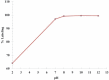
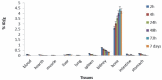
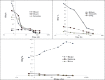
Methods: TTHMP was synthesized and characterized, using spectroscopic methods. Radiochemical purity of the (177)Lu-TTHMP complex was determined using instant thin-layer chromatography (ITLC) and high performance liquid chromatography (HPLC) methods. The complex was injected to wild-type rats and biodistribution was studied for 7 days. Preliminary dose evaluation was investigated based on biodistribution data in rats.
Results: (177)Lu was prepared with 2.6-3 GBq/mg specific activity and radionuclide purity of 99.98%. (177)Lu-TTHMP was successfully prepared with high radiochemical purity (>99%). The complex showed rapid bone uptake, while accumulation in other organs was insignificant. Dosimetric results showed that all tissues received almost insignificant absorbed doses in comparison with bone tissues.
Conclusion: Based on the obtained results, this radiopharmaceutical can be a good candidate for bone pain palliation therapy in skeletal metastases.
Keywords: Bone pain palliation; Dosimetry; Lu-177; TTHMP.
Figures

Similar articles
-
(175)Yb-TTHMP as a good candidate for bone pain palliation and substitute of other radiopharmaceuticals.Indian J Nucl Med. 2014 Jul;29(3):135-9. doi: 10.4103/0972-3919.136555. Indian J Nucl Med. 2014. PMID: 25210277 Free PMC article.
-
Studies on 177Lu-labeled methylene diphosphonate as potential bone-seeking radiopharmaceutical for bone pain palliation.Nucl Med Biol. 2011 Apr;38(3):417-25. doi: 10.1016/j.nucmedbio.2010.09.013. Epub 2010 Dec 3. Nucl Med Biol. 2011. PMID: 21492790
-
Production, quality control, biodistribution assessment and preliminary dose evaluation of (177)Lu-PDTMP as a possible bone palliative agent.Nucl Med Commun. 2014 Jan;35(1):99-107. doi: 10.1097/MNM.0000000000000018. Nucl Med Commun. 2014. PMID: 24162837
-
177Lu-Labeled sodium pyrophosphate.2012 Nov 27 [updated 2012 Dec 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Nov 27 [updated 2012 Dec 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23256226 Free Books & Documents. Review.
-
Production of (177)Lu for Targeted Radionuclide Therapy: Available Options.Nucl Med Mol Imaging. 2015 Jun;49(2):85-107. doi: 10.1007/s13139-014-0315-z. Epub 2015 Feb 17. Nucl Med Mol Imaging. 2015. PMID: 26085854 Free PMC article. Review.
Cited by
-
Production and quality control 177Lu (NCA)-DOTMP as a potential agent for bone pain palliation.J Appl Clin Med Phys. 2016 Nov 8;17(6):128-139. doi: 10.1120/jacmp.v17i6.6375. J Appl Clin Med Phys. 2016. PMID: 27929488 Free PMC article.
-
Evaluation of the Possible Utilization of 68Ga-DOTATOC in Diagnosis of Adenocarcinoma Breast Cancer.Asia Ocean J Nucl Med Biol. 2018 Winter;6(1):41-49. doi: 10.22038/aojnmb.2017.23695.1168. Asia Ocean J Nucl Med Biol. 2018. PMID: 29333466 Free PMC article.
References
-
- Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906. - PubMed
-
- Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical Therapy for Palliation of Bone Pain from Osseous Metastases. J Nucl Med. 2004;45:1358–65. - PubMed
-
- Austria, Vienna: IAEA; 2007. Criteria for Palliation of Bone Metastases – Clinical Applications, IAEA-TECDOC-1549.
-
- Lipton A. Pathophysiology of Bone Metastases: How This Knowledge May Lead to Therapeutic Intervention. J Support Oncol. 2004;2:205–13. - PubMed
-
- Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain Palliation in Patients with Bone Metastases Using MR-Guided Focused Ultrasound Surgery: A Multicenter Study. Ann Surg Oncol. 2009;16:140–6. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
