Physiologically Based Pharmacokinetic (PBPK) Model for Biodistribution of Radiolabeled Peptides in Patients with Neuroendocrine Tumours
- PMID: 27408897
- PMCID: PMC4938879
- DOI: 10.7508/aojnmb.2016.02.005
Physiologically Based Pharmacokinetic (PBPK) Model for Biodistribution of Radiolabeled Peptides in Patients with Neuroendocrine Tumours
Abstract
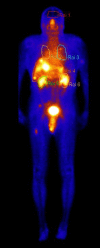
Objectives: The objectives of this work was to assess the benefits of the application of Physiologically Based Pharmacokinetic (PBPK) models in patients with different neuroendocrine tumours (NET) who were treated with Lu-177 DOTATATE. The model utilises clinical data on biodistribution of radiolabeled peptides (RLPs) obtained by whole body scintigraphy (WBS) of the patients.
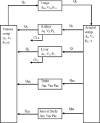
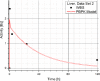
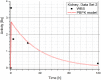
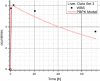
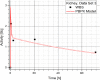
Methods: The blood flow restricted (perfusion rate limited) type of the PBPK model for biodistribution of radiolabeled peptides (RLPs) in individual human organs is based on the multi-compartment approach, which takes into account the main physiological processes in the organism: absorption, distribution, metabolism and excretion (ADME). The approach calibrates the PBPK model for each patient in order to increase the accuracy of the dose estimation. Datasets obtained using WBS in four patients have been used to obtain the unknown model parameters. The scintigraphic data were acquired using a double head gamma camera in patients with different neuroendocrine tumours who were treated with Lu-177 DOTATATE. The activity administered to each patient was 7400 MBq.
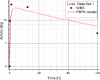
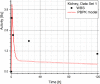
Results: Satisfactory agreement of the model predictions with the data obtained from the WBS for each patient has been achieved.
Conclusion: The study indicates that the PBPK model can be used for more accurate calculation of biodistribution and absorbed doses in patients. This approach is the first attempt of utilizing scintigraphic data in PBPK models, which was obtained during Lu-177 peptide therapy of patients with NET.
Keywords: Lu-177 DOTATATE; PBPK model; Radiolabeled peptides; Whole body scintigraphy.
Figures
References
-
- Theil FP, Guentert TW, Haddad S, Poulin P. Utility of physiologically based pharmacokinetic models to drug development and rational drug discovery candidate selection. Toxicol Lett. 2003;138(1-2):29–49. - PubMed
-
- Lee HA, Leavens TL, Mason SE, Monteiro-Riviere NA, Riviere JE. Comparison of quantum dot biodistribution with a blood-flow-limited physiologically based pharmacokinetic model. Nano Lett. 2009;9(2):794–9. - PubMed
-
- Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies:scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–22. - PubMed
-
- Baxter LT, Zhu H, Mackensen DG, Jain RK. physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54(6):1517–28. - PubMed
-
- Adams JC, Dills RL, Morgan MS, Kalman DA, Pierce CH. A physiologically based toxicokinetic model of inhalation exposure to xylenes in Caucasian men. Regul Toxicol Pharmacol. 2005;43(2):203–14. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
