The Use and Reporting of the Cross-Over Study Design in Clinical Trials and Systematic Reviews: A Systematic Assessment
- PMID: 27409076
- PMCID: PMC4943623
- DOI: 10.1371/journal.pone.0159014
The Use and Reporting of the Cross-Over Study Design in Clinical Trials and Systematic Reviews: A Systematic Assessment
Abstract
Background: Systematic reviews of treatment interventions in stable or chronic conditions often require the synthesis of clinical trials with a cross-over design. Previous work has indicated that methodology for analysing cross-over data is inadequate in trial reports and in systematic reviews assessing trials with this design.
Objective: We assessed systematic review methodology for synthesising cross-over trials among Cochrane Cystic Fibrosis and Genetic Disorders Group reviews published to July 2015, and assessed the quality of reporting among the cross-over trials included in these reviews.
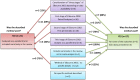
Methodology: We performed data extraction of methodology and reporting in reviews, trials identified and trials included within reviews.
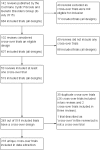
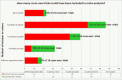
Principal findings: We reviewed a total of 142 Cochrane systematic reviews including 53 reviews which synthesised evidence from 218 cross-over trials. Thirty-three (63%) Cochrane reviews described a clear and appropriate method for the inclusion of cross-over data, and of these 19 (56%) used the same method to analyse results. 145 cross-over trials were described narratively or treated as parallel trials in reviews but in 30 (21%) of these trials data existed in the trial reports to account for the cross-over design. At the trial level, the analysis and presentation of results were often inappropriate or unclear, with only 69 (32%) trials presenting results that could be included in meta-analysis.
Conclusions: Despite development of accessible, technical guidance and training for Cochrane systematic reviewers, statistical analysis and reporting of cross-over data is inadequate at both the systematic review and the trial level. Plain language and practical guidance for the inclusion of cross-over data in meta-analysis would benefit systematic reviewers, who come from a wide range of health specialties. Minimum reporting standards for cross-over trials are needed.
Conflict of interest statement
Figures

References
-
- Senn SJ. Cross-over trials in clinical research Chichester: John Wiley. 2002
-
- Brown BW Jr. The crossover experiment for clinical trials. Biometrics. 1980; 36: 69–79. - PubMed
-
- Louis TA, Lavori PW, Bailar JC, Polansky M. Crossover and self-controlled designs in clinical research. NEJM 1984, 310:24–31. - PubMed
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epid. 2002; 31:140–149. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

