Optical Coherence Tomography and the Development of Antiangiogenic Therapies in Neovascular Age-Related Macular Degeneration
- PMID: 27409464
- PMCID: PMC4968916
- DOI: 10.1167/iovs.16-19969
Optical Coherence Tomography and the Development of Antiangiogenic Therapies in Neovascular Age-Related Macular Degeneration
Abstract
Purpose: To explain the pivotal role optical coherence tomography (OCT) imaging had in the development of antiangiogenic therapies for the treatment of neovascular age-related macular degeneration (nvAMD).
Methods: A historical literature review was combined with personal perspectives from the introduction of OCT imaging and the early clinical use of vascular endothelial growth factor (VEGF) inhibitors.
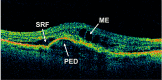
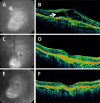
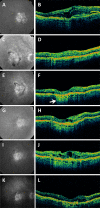
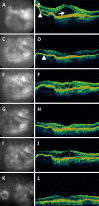
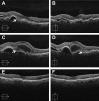
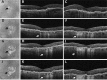
Results: At the time that OCT emerged, the gold standard for imaging of nvAMD was fluorescein angiography (FA), a time-consuming, dye-based, invasive technique that provided en face images of the retina and was used to characterize leakage, perfusion status, and the types of macular neovascularization (MNV). In comparison, OCT imaging was a fast, safe, noninvasive technique that complemented FA imaging by providing cross-sectional images of the macula. OCT was able to visualize and quantify the macular fluid that was associated with the presence of excess VEGF, which was identified by intraretinal fluid, subretinal fluid, and fluid under the retinal pigment epithelium (RPE). Clinicians quickly appreciated the benefits of OCT imaging for following macular fluid after anti-VEGF therapy. By observing the qualitative and quantitative changes in macular fluid depicted by OCT imaging, clinicians were empowered to compare anti-VEGF drugs and move from fixed-dosing regimens to patient-specific dosing strategies requiring fewer injections.
Conclusions: Optical coherence tomography imaging was adopted as a VEGF-meter, a method to detect excess VEGF, and evolved to become the gold standard imaging strategy for diagnosing nvAMD, assessing treatment responses to anti-VEGF drugs, deciding when to re-treat, and evaluating disease progression.
Figures
References
-
- Gass JDM. Stereoscopic Atlas of Macular Disease. Diagnosis and Treatment. 4th ed. St. Louis: Mosby-Year Book, Inc.; 1997: 130–147.
-
- Puliafito CA,, Schuman JS,, Hee MR,, Fujimoto JG. Optical Coherence Tomography of Ocular Diseases. 2nd ed. Thorofare, NJ: Slack Inc, 2004.
-
- Staurenghi G,, Sadda S,, Chakravarthy U,, Spaide RF;, International Nomenclature for Optical Coherence Tomography Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014; 121: 1572–1578. - PubMed
-
- Ting TD,, Oh M,, Cox TA,, Meyer CH,, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol. 2002; 120: 731–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

