Retinal Structure and Gene Therapy Outcome in Retinoschisin-Deficient Mice Assessed by Spectral-Domain Optical Coherence Tomography
- PMID: 27409484
- PMCID: PMC4968785
- DOI: 10.1167/iovs.15-18920
Retinal Structure and Gene Therapy Outcome in Retinoschisin-Deficient Mice Assessed by Spectral-Domain Optical Coherence Tomography
Abstract
Purpose: Spectral-domain optical coherence tomography (SD-OCT) was used to characterize the retinal phenotype, natural history, and treatment responses in a mouse model of X-linked retinoschisis (Rs1-KO) and to identify new structural markers of AAV8-mediated gene therapy outcome.
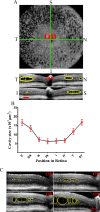
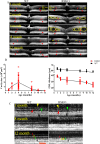
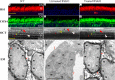
Methods: Optical coherence tomography scans were performed on wild-type and Rs1-KO mouse retinas between 1 and 12 months of age and on Rs1-KO mice after intravitreal injection of AAV8-scRS/IRBPhRS (AAV8-RS1). Cavities and photoreceptor outer nuclear layer (ONL) thickness were measured, and outer retina reflective band (ORRB) morphology was examined with age and after AAV8-RS1 treatment. Outer retina reflective band morphology was compared to immunohistochemical staining of the outer limiting membrane (OLM) and photoreceptor inner segment (IS) mitochondria and to electron microscopy (EM) images of IS.
Results: Retinal cavity size in Rs1-KO mice increased between 1 and 4 months and decreased thereafter, while ONL thickness declined steadily, comparable to previous histologic studies. Wild-type retina had four ORRBs. In Rs1-KO, ORRB1was fragmented from 1 month, but was normal after 8 months; ORRB2 and ORRB3 were merged at all ages. Outer retina reflective band morphology returned to normal after AAV-RS1 therapy, paralleling the recovery of the OLM and IS mitochondria as indicated by anti-β-catenin and anti-COX4 labeling, respectively, and EM.
Conclusions: Spectral-domain OCT is a sensitive, noninvasive tool to monitor subtle changes in retinal morphology, disease progression, and effects of therapies in mouse models. The ORRBs may be useful to assess the outcome of gene therapy in the treatment of X-linked retinoschisis patients.
Figures



References
-
- Mooy CM,, Van Den Born LI,, Baarsma S,, et al. Hereditary X-linked juvenile retinoschisis: a review of the role of Müller cells. Arch Ophthalmol. 2002; 120: 979–984. - PubMed
-
- Azzolini C,, Pierro L,, Codenotti M,, Brancato R. OCT images and surgery of juvenile macular retinoschisis. Eur J Ophthalmol. 1997; 7: 196–200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

