Optical Coherence Tomography for Retinal Surgery: Perioperative Analysis to Real-Time Four-Dimensional Image-Guided Surgery
- PMID: 27409495
- PMCID: PMC4968921
- DOI: 10.1167/iovs.16-19277
Optical Coherence Tomography for Retinal Surgery: Perioperative Analysis to Real-Time Four-Dimensional Image-Guided Surgery
Abstract
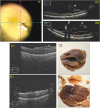
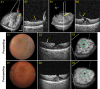
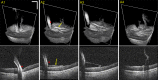
Magnification of the surgical field using the operating microscope facilitated profound innovations in retinal surgery in the 1970s, such as pars plana vitrectomy. Although surgical instrumentation and illumination techniques are continually developing, the operating microscope for vitreoretinal procedures has remained essentially unchanged and currently limits the surgeon's depth perception and assessment of subtle microanatomy. Optical coherence tomography (OCT) has revolutionized clinical management of retinal pathology, and its introduction into the operating suite may have a similar impact on surgical visualization and treatment. In this article, we review the evolution of OCT for retinal surgery, from perioperative analysis to live volumetric (four-dimensional, 4D) image-guided surgery. We begin by briefly addressing the benefits and limitations of the operating microscope, the progression of OCT technology, and OCT applications in clinical/perioperative retinal imaging. Next, we review intraoperative OCT (iOCT) applications using handheld probes during surgical pauses, two-dimensional (2D) microscope-integrated OCT (MIOCT) of live surgery, and volumetric MIOCT of live surgery. The iOCT discussion focuses on technological advancements, applications during human retinal surgery, translational difficulties and limitations, and future directions.
Figures


References
-
- Daniel RK. Microsurgery: through the looking glass. N Engl J Med. 1979; 300: 1251–1257. - PubMed
-
- Barraquer J,, Barraquer J,, Littman HA. New operating microscope for ocular surgery. Am J Ophthalmol. 1967; 63: 90–97. - PubMed
-
- Harms H,, Mackensen G. Ocular Surgery Under the Microscope. Chicago: Year Book Medical Publishers; 1967.
-
- Machemer R,, Buettner H,, Norton E,, Parel J. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol. 1971; 75: 813–820. - PubMed
-
- Machemer R. The development of pars plana vitrectomy: a personal account. Graefes Arch Clin Exp Ophthalmol. 1995; 233: 453–468. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

