Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances
- PMID: 27409596
- PMCID: PMC6274578
- DOI: 10.3390/molecules21070900
Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances
Abstract
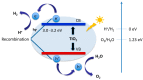
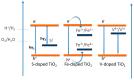
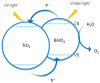
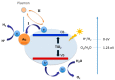
Photocatalytic water splitting using sunlight is a promising technology capable of providing high energy yield without pollutant byproducts. Herein, we review various aspects of this technology including chemical reactions, physiochemical conditions and photocatalyst types such as metal oxides, sulfides, nitrides, nanocomposites, and doped materials followed by recent advances in computational modeling of photoactive materials. As the best-known catalyst for photocatalytic hydrogen and oxygen evolution, TiO₂ is discussed in a separate section, along with its challenges such as the wide band gap, large overpotential for hydrogen evolution, and rapid recombination of produced electron-hole pairs. Various approaches are addressed to overcome these shortcomings, such as doping with different elements, heterojunction catalysts, noble metal deposition, and surface modification. Development of a photocatalytic corrosion resistant, visible light absorbing, defect-tuned material with small particle size is the key to complete the sunlight to hydrogen cycle efficiently. Computational studies have opened new avenues to understand and predict the electronic density of states and band structure of advanced materials and could pave the way for the rational design of efficient photocatalysts for water splitting. Future directions are focused on developing innovative junction architectures, novel synthesis methods and optimizing the existing active materials to enhance charge transfer, visible light absorption, reducing the gas evolution overpotential and maintaining chemical and physical stability.
Keywords: hydrogen; metal oxides; nanomaterials; nanotechnology; photocatalysis; photocatalysts; semiconductors; solar fuels; water splitting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pao H.-T., Tsai C.-M. CO2 emissions, energy consumption and economic growth in BRIC countries. Energy Policy. 2010;38:7850–7860. doi: 10.1016/j.enpol.2010.08.045. - DOI
-
- Dodman D. Blaming cities for climate change? An analysis of urban greenhouse gas emissions inventories. Environ. Urban. 2009;21:185–201. doi: 10.1177/0956247809103016. - DOI
-
- Moniz S., Shevlin S.A., Martin D., Guo Z., Tang J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting—A Critical Review. Energy Environ. Sci. 2015;8:731–759. doi: 10.1039/C4EE03271C. - DOI
-
- Byrne J., Hughes K., Rickerson W., Kurdgelashvili L. American policy conflict in the greenhouse: Divergent trends in federal, regional, state, and local green energy and climate change policy. Energy Policy. 2007;35:4555–4573. doi: 10.1016/j.enpol.2007.02.028. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

