Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends
- PMID: 27409609
- PMCID: PMC4964472
- DOI: 10.3390/ijms17071096
Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends
Abstract
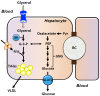
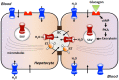
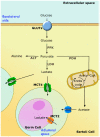
Aquaporins (AQPs) are proteinaceous channels widespread in nature where they allow facilitated permeation of water and uncharged through cellular membranes. AQPs play a number of important roles in both health and disease. This review focuses on the most recent advances and research trends regarding the expression and modulation, as well as physiological and pathophysiological functions of AQPs in hepatocytes and Sertoli cells (SCs). Besides their involvement in bile formation, hepatocyte AQPs are involved in maintaining energy balance acting in hepatic gluconeogenesis and lipid metabolism, and in critical processes such as ammonia detoxification and mitochondrial output of hydrogen peroxide. Roles are played in clinical disorders including fatty liver disease, diabetes, obesity, cholestasis, hepatic cirrhosis and hepatocarcinoma. In the seminiferous tubules, particularly in SCs, AQPs are also widely expressed and seem to be implicated in the various stages of spermatogenesis. Like in hepatocytes, AQPs may be involved in maintaining energy homeostasis in these cells and have a major role in the metabolic cooperation established in the testicular tissue. Altogether, this information represents the mainstay of current and future investigation in an expanding field.
Keywords: Non-Alcoholic Fatty Liver Disease (NAFLD); bile formation; liver; male fertility; metabolic homeostasis; mitochondria; reactive oxygen species (ROS); spermatogenesis; testis.
Figures


References
-
- Calamita G., Delporte C., Marinelli R.A. Hepatobiliary, salivary glands and pancreas aquaporins in health and disease. In: Soveral G., Casini A., Nielsen S., editors. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2015. pp. 193–196. Chapter 9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

