Expression of the Bovine NK-Lysin Gene Family and Activity against Respiratory Pathogens
- PMID: 27409794
- PMCID: PMC4943647
- DOI: 10.1371/journal.pone.0158882
Expression of the Bovine NK-Lysin Gene Family and Activity against Respiratory Pathogens
Abstract
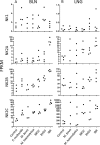
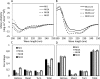
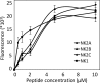
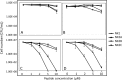
Unlike the genomes of many mammals that have a single NK-lysin gene, the cattle genome contains a family of four genes, one of which is expressed preferentially in the lung. In this study, we compared the expression of the four bovine NK-lysin genes in healthy animals to animals challenged with pathogens known to be associated with bovine respiratory disease (BRD) using transcriptome sequencing (RNA-seq). The expression of several NK-lysins, especially NK2C, was elevated in challenged relative to control animals. The effects of synthetic peptides corresponding to functional region helices 2 and 3 of each gene product were tested on both model membranes and bio-membranes. Circular dichroism spectroscopy indicated that these peptides adopted a more helical secondary structure upon binding to an anionic model membrane and liposome leakage assays suggested that these peptides disrupt membranes. Bacterial killing assays further confirmed the antimicrobial effects of these peptides on BRD-associated bacteria, including both Pasteurella multocida and Mannhemia haemolytica and an ultrastructural examination of NK-lysin-treated P. multocida cells by transmission electron microscopy revealed the lysis of target membranes. These studies demonstrate that the expanded bovine NK-lysin gene family is potentially important in host defense against pathogens involved in bovine respiratory disease.
Conflict of interest statement
Figures
Similar articles
-
Antimicrobial activity of bovine NK-lysin-derived peptides on bovine respiratory pathogen Histophilus somni.PLoS One. 2017 Aug 21;12(8):e0183610. doi: 10.1371/journal.pone.0183610. eCollection 2017. PLoS One. 2017. PMID: 28827826 Free PMC article.
-
Antimicrobial activity of bovine NK-lysin-derived peptides on Mycoplasma bovis.PLoS One. 2018 May 17;13(5):e0197677. doi: 10.1371/journal.pone.0197677. eCollection 2018. PLoS One. 2018. PMID: 29771981 Free PMC article.
-
Multidrug resistant Mannheimia haemolytica isolated from high-risk beef stocker cattle after antimicrobial metaphylaxis and treatment for bovine respiratory disease.Vet Microbiol. 2018 Jul;221:143-152. doi: 10.1016/j.vetmic.2018.06.005. Epub 2018 Jun 8. Vet Microbiol. 2018. PMID: 29981701
-
Mannheimia haemolytica and Pasteurella multocida in Bovine Respiratory Disease: How Are They Changing in Response to Efforts to Control Them?Vet Clin North Am Food Anim Pract. 2020 Jul;36(2):253-268. doi: 10.1016/j.cvfa.2020.02.001. Epub 2020 Apr 21. Vet Clin North Am Food Anim Pract. 2020. PMID: 32327253 Review.
-
A literature review of antimicrobial resistance in Pathogens associated with bovine respiratory disease.Anim Health Res Rev. 2015 Dec;16(2):125-34. doi: 10.1017/S146625231500016X. Epub 2015 Sep 16. Anim Health Res Rev. 2015. PMID: 26373635 Review.
Cited by
-
Comprehensive time-course gene expression evaluation of high-risk beef cattle to establish immunological characteristics associated with undifferentiated bovine respiratory disease.Front Immunol. 2024 Sep 13;15:1412766. doi: 10.3389/fimmu.2024.1412766. eCollection 2024. Front Immunol. 2024. PMID: 39346910 Free PMC article.
-
Whole-Blood Transcriptome Analysis of Feedlot Cattle With and Without Bovine Respiratory Disease.Front Genet. 2021 Mar 8;12:627623. doi: 10.3389/fgene.2021.627623. eCollection 2021. Front Genet. 2021. PMID: 33763112 Free PMC article.
-
The Immunology of Bovine Respiratory Disease: Recent Advancements.Vet Clin North Am Food Anim Pract. 2020 Jul;36(2):333-348. doi: 10.1016/j.cvfa.2020.03.002. Epub 2020 Apr 21. Vet Clin North Am Food Anim Pract. 2020. PMID: 32327252 Free PMC article. Review.
-
Strategies for Bovine Respiratory Disease (BRD) Diagnosis and Prognosis: A Comprehensive Overview.Animals (Basel). 2024 Feb 16;14(4):627. doi: 10.3390/ani14040627. Animals (Basel). 2024. PMID: 38396598 Free PMC article. Review.
-
Synthetic bovine NK-lysin-derived peptide (bNK2A) does not require intra-chain disulfide bonds for bactericidal activity.PLoS One. 2019 Jun 19;14(6):e0218507. doi: 10.1371/journal.pone.0218507. eCollection 2019. PLoS One. 2019. PMID: 31216348 Free PMC article.
References
-
- Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. Journal of immunology (Baltimore, Md: 1950). 1997;158(6):2680–8. Epub 1997/03/15. . - PubMed
-
- Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Sillard R, et al. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. The EMBO journal. 1995;14(8):1615–25. Epub 1995/04/18. ; PubMed Central PMCID: PMCPmc398254. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

