Lgr5+ stem cells and their progeny in mouse epidermis under regimens of exogenous skin carcinogenesis, and their absence in ensuing skin tumors
- PMID: 27409834
- PMCID: PMC5239536
- DOI: 10.18632/oncotarget.10475
Lgr5+ stem cells and their progeny in mouse epidermis under regimens of exogenous skin carcinogenesis, and their absence in ensuing skin tumors
Abstract
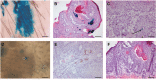
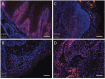
Actively proliferating Lgr5+ skin stem cells are found deep in the hair follicle (HF). These cells renew the HF and drive its expansion in anagen phase. Their long residence and continuous mitotic activity make them prime candidates to transform into skin tumor-initiating cells. This was investigated by subjecting Lgr5-EGFP-Ires-CreERT2/R26R-LacZ mice (haired and hairless) to chemical and UV carcinogenic regimens. In the course of these regimens Lgr5+ cells (EGFP+) remained exclusively located in HFs, and in deep-seated cysts of hairless skin. In haired mice, progeny of Lgr5+ stem cells (LacZ+ after a pulse of tamoxifen) appeared in the interfollicular epidermis upon UV-induced sunburn and in TPA-induced hyperplasia. In hairless mice the progeny remained located in deep-seated cysts and in HF remnants. Progeny in hairless skin was only detected interfollicularly at a late stage, in between outgrowing tumors. Lgr5+ stem cells were absent in the ultimate tumor masses, and no tumor appeared to be a (clonal) expansion of Lgr5+ cells (52 tumors with tamoxifen at the start of carcinogenesis, 42 tumors with tamoxifen late during tumor outgrowth). In contrast to CD34/K15+ quiescent bulge stem cells, actively proliferating Lgr5+ stem cells do therefore not appear to be tumor drivers in experimental skin carcinogenesis.
Keywords: Lgr5; UV; lineage tracing; skin carcinogenesis; stem cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Lgr6+ stem cells and their progeny in mouse epidermis under regimens of exogenous skin carcinogenesis, and their absence in ensuing skin tumors.Oncotarget. 2016 Dec 27;7(52):86740-86754. doi: 10.18632/oncotarget.13436. Oncotarget. 2016. PMID: 27880932 Free PMC article.
-
Progeny of Lgr5-expressing hair follicle stem cell contributes to papillomavirus-induced tumor development in epidermis.Oncogene. 2013 Aug 8;32(32):3732-43. doi: 10.1038/onc.2012.375. Epub 2012 Sep 3. Oncogene. 2013. PMID: 22945646
-
Fractionation of a tumor-initiating UV dose introduces DNA damage-retaining cells in hairless mouse skin and renders subsequent TPA-promoted tumors non-regressing.Oncotarget. 2016 Feb 16;7(7):8067-77. doi: 10.18632/oncotarget.6932. Oncotarget. 2016. PMID: 26797757 Free PMC article.
-
Wnt signaling, lgr5, and stem cells in the intestine and skin.Am J Pathol. 2009 Mar;174(3):715-21. doi: 10.2353/ajpath.2009.080758. Epub 2009 Feb 5. Am J Pathol. 2009. PMID: 19197002 Free PMC article. Review.
-
Early events in UV carcinogenesis--DNA damage, target cells and mutant p53 foci.Photochem Photobiol. 2008 Mar-Apr;84(2):382-7. doi: 10.1111/j.1751-1097.2007.00275.x. Epub 2008 Jan 23. Photochem Photobiol. 2008. PMID: 18221455 Review.
Cited by
-
The Use of Retinoids for the Prevention and Treatment of Skin Cancers: An Updated Review.Int J Mol Sci. 2022 Oct 20;23(20):12622. doi: 10.3390/ijms232012622. Int J Mol Sci. 2022. PMID: 36293471 Free PMC article. Review.
-
IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells.J Exp Med. 2019 Jan 7;216(1):195-214. doi: 10.1084/jem.20171849. Epub 2018 Dec 21. J Exp Med. 2019. PMID: 30578323 Free PMC article.
-
Method to Study Skin Cancer: Two-Stage Chemically Induced Carcinogenesis in Mouse Skin.Methods Mol Biol. 2020;2154:231-238. doi: 10.1007/978-1-0716-0648-3_19. Methods Mol Biol. 2020. PMID: 32314221
-
Integrin α3β1 in hair bulge stem cells modulates CCN2 expression and promotes skin tumorigenesis.Life Sci Alliance. 2020 May 18;3(7):e202000645. doi: 10.26508/lsa.202000645. Print 2020 Jul. Life Sci Alliance. 2020. PMID: 32423907 Free PMC article.
-
Pathogenesis of Skin Carcinomas and a Stem Cell as Focal Origin.Front Med (Lausanne). 2018 May 29;5:165. doi: 10.3389/fmed.2018.00165. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29896477 Free PMC article. Review.
References
-
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

