Loss of FBXW7 and accumulation of MCL1 and PLK1 promote paclitaxel resistance in breast cancer
- PMID: 27409838
- PMCID: PMC5288146
- DOI: 10.18632/oncotarget.10481
Loss of FBXW7 and accumulation of MCL1 and PLK1 promote paclitaxel resistance in breast cancer
Abstract
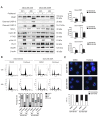
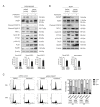
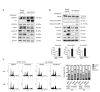
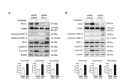
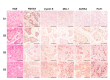
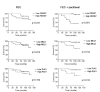
FBXW7 is a component of SCF (complex of SKP1, CUL1 and F-box-protein)-type ubiquitin ligases that targets several oncoproteins for ubiquitination and degradation by the proteasome. FBXW7 regulates cellular apoptosis by targeting MCL1 for ubiquitination. Recently, we identified PLK1 as a new substrate of FBXW7 modulating the intra-S-phase DNA-damage checkpoint. Taxanes are frequently used in breast cancer treatments, but the acquisition of resistance makes these treatments ineffective. We investigated the role of FBXW7 and their substrates MCL1 and PLK1 in regulating the apoptotic response to paclitaxel treatment in breast cancer cells and their expression in breast cancer tissues. Paclitaxel-sensitive MDA-MB-468 and a paclitaxel-resistant MDA-MB-468R subclone were used to study the role of FBXW7 and substrates in paclitaxel-induced apoptosis. Forced expression of FBXW7 or downregulation of MCL1 or PLK1 restored sensitivity to paclitaxel in MDA-MB-468R cells. By contrary, FBXW7-silenced MDA-MB-468 cells became resistant to paclitaxel. The expression of FBXW7 and substrates were studied in 296 invasive carcinomas by immunohistochemistry and disease-free survival was analyzed in a subset of patients treated with paclitaxel. In breast cancer tissues, loss of FBXW7 correlated with adverse prognosis markers and loss of FBXW7 and MCL1 or PLK1 accumulation were associated with diminished disease-free survival in paclitaxel-treated patients. We conclude that FBXW7 regulates the response to paclitaxel by targeting MCL1 and PLK1 in breast cancer cells and thus targeting these substrates may be a valuable adjunct for paclitaxel treatment. Also, FBXW7, MCL1 and PLK1 may be relevant predictive markers of tumor progression and response to paclitaxel treatment.
Keywords: FBXW7; MCL1; PLK1; Pathology Section; apoptosis; paclitaxel.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

Similar articles
-
Rational combinations of siRNAs targeting Plk1 with breast cancer drugs.Oncogene. 2007 Aug 23;26(39):5793-807. doi: 10.1038/sj.onc.1210355. Epub 2007 Mar 19. Oncogene. 2007. PMID: 17369857
-
Down-regulation of Polo-like kinase 1 elevates drug sensitivity of breast cancer cells in vitro and in vivo.Cancer Res. 2006 Jun 1;66(11):5836-46. doi: 10.1158/0008-5472.CAN-06-0343. Cancer Res. 2006. PMID: 16740723
-
FBXW7 mediates chemotherapeutic sensitivity and prognosis in NSCLCs.Mol Cancer Res. 2014 Jan;12(1):32-7. doi: 10.1158/1541-7786.MCR-13-0341. Epub 2013 Oct 28. Mol Cancer Res. 2014. PMID: 24165483
-
The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues.Cancer Rep (Hoboken). 2021 Aug;4(4):e1369. doi: 10.1002/cnr2.1369. Epub 2021 Apr 6. Cancer Rep (Hoboken). 2021. PMID: 33822486 Free PMC article. Review.
-
Recent insight into the role of FBXW7 as a tumor suppressor.Semin Cancer Biol. 2020 Dec;67(Pt 2):1-15. doi: 10.1016/j.semcancer.2020.02.017. Epub 2020 Feb 27. Semin Cancer Biol. 2020. PMID: 32113998 Review.
Cited by
-
Identification of a mechanogenetic link between substrate stiffness and chemotherapeutic response in breast cancer.Biomaterials. 2019 May;202:1-11. doi: 10.1016/j.biomaterials.2019.02.018. Epub 2019 Feb 19. Biomaterials. 2019. PMID: 30818087 Free PMC article.
-
E3 Ubiquitin Ligase in Anticancer Drugdsla Resistance: Recent Advances and Future Potential.Front Pharmacol. 2021 Apr 15;12:645864. doi: 10.3389/fphar.2021.645864. eCollection 2021. Front Pharmacol. 2021. PMID: 33935743 Free PMC article. Review.
-
Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by suppressing miR-155 expression and activating FBXW7 function.Am J Cancer Res. 2016 Nov 1;6(11):2561-2574. eCollection 2016. Am J Cancer Res. 2016. PMID: 27904771 Free PMC article.
-
Genomic and Immune Profiling of a Patient With Triple-Negative Breast Cancer That Progressed During Neoadjuvant Chemotherapy Plus PD-L1 Blockade.JCO Precis Oncol. 2019 May 10;3:PO.18.00335. doi: 10.1200/PO.18.00335. eCollection 2019. JCO Precis Oncol. 2019. PMID: 32914041 Free PMC article. No abstract available.
-
Obatoclax and Paclitaxel Synergistically Induce Apoptosis and Overcome Paclitaxel Resistance in Urothelial Cancer Cells.Cancers (Basel). 2018 Dec 5;10(12):490. doi: 10.3390/cancers10120490. Cancers (Basel). 2018. PMID: 30563080 Free PMC article.
References
-
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. - PubMed
-
- Tan Y, Sangfelt O, Spruck C. The Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett. 2008;271:1–12. - PubMed
-
- Akhoondi S, Sun D, Von Der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dofou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, Nordgren H, Grander D, Reed SI, Widschwendter M, Sangfelt O, Spruck C. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

