Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model
- PMID: 27410049
- PMCID: PMC4968716
- DOI: 10.1073/pnas.1608873113
Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model
Abstract
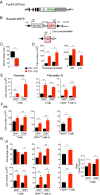
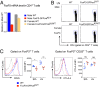
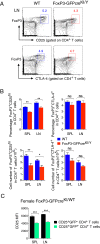
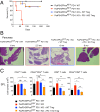
PD-1 (programmed-death 1), an immune-inhibitory receptor required for immune self-tolerance whose deficiency causes autoimmunity with variable severity and tissue specificity depending on other genetic factors, is expressed on activated T cells, including the transcription factor FoxP3(+) Treg cells known to play critical roles in maintaining immune tolerance. However, whether PD-1 expression by the Treg cells is required for their immune regulatory function, especially in autoimmune settings, is still unclear. We found that mice with partial FoxP3 insufficiency developed early-onset lympho-proliferation and lethal autoimmune pancreatitis only when PD-1 is absent. The autoimmune phenotype was rescued by the transfer of FoxP3-sufficient T cells, regardless of whether they were derived from WT or PD-1-deficient mice, indicating that Treg cells dominantly protect against development of spontaneous autoimmunity without intrinsic expression of PD-1. The absence of PD-1 combined with partial FoxP3 insufficiency, however, led to generation of ex-FoxP3 T cells with proinflammatory properties and expansion of effector/memory T cells that contributed to the autoimmune destruction of target tissues. Altogether, the results suggest that PD-1 and FoxP3 work collaboratively in maintaining immune tolerance mostly through nonoverlapping pathways. Thus, PD-1 is modulating the activation threshold and maintaining the balance between regulatory and effector T cells, whereas FoxP3 is sufficient for dominant regulation through maintaining the integrity of the Treg function. We suggest that genetic or environmental factors that even moderately affect the expression of both PD-1 and FoxP3 can cause life-threatening autoimmune diseases by disrupting the T-cell homeostasis.
Keywords: PD-1; T lymphocytes; autoimmunity; immune tolerance; regulatory T cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Membrane-bound Dickkopf-1 in Foxp3+ regulatory T cells suppresses T-cell-mediated autoimmune colitis.Immunology. 2017 Oct;152(2):265-275. doi: 10.1111/imm.12766. Epub 2017 Jun 27. Immunology. 2017. PMID: 28556921 Free PMC article.
-
Few Foxp3⁺ regulatory T cells are sufficient to protect adult mice from lethal autoimmunity.Eur J Immunol. 2014 Oct;44(10):2990-3002. doi: 10.1002/eji.201344315. Epub 2014 Aug 11. Eur J Immunol. 2014. PMID: 25042334
-
Critical co-stimulatory pathways in the stability of Foxp3+ Treg cell homeostasis in Type I diabetes.Autoimmun Rev. 2011 Dec;11(2):104-11. doi: 10.1016/j.autrev.2011.08.007. Epub 2011 Aug 22. Autoimmun Rev. 2011. PMID: 21875694 Review.
-
CD8+ T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a,b-/- mice favoring autoimmune cholangitis.Oncotarget. 2015 Oct 6;6(30):28588-606. doi: 10.18632/oncotarget.5665. Oncotarget. 2015. PMID: 26396175 Free PMC article.
-
The PD-1 pathway in tolerance and autoimmunity.Immunol Rev. 2010 Jul;236:219-42. doi: 10.1111/j.1600-065X.2010.00923.x. Immunol Rev. 2010. PMID: 20636820 Free PMC article. Review.
Cited by
-
Immune checkpoint expression patterns on T cell subsets in light-chain amyloidosis: VISTA, PD-1, and TIGIT as potential therapeutic targets.Blood Sci. 2024 Jan 15;6(1):e00181. doi: 10.1097/BS9.0000000000000181. eCollection 2024 Jan. Blood Sci. 2024. PMID: 38226018 Free PMC article.
-
Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy.Cancers (Basel). 2020 Oct 6;12(10):2870. doi: 10.3390/cancers12102870. Cancers (Basel). 2020. PMID: 33036192 Free PMC article. Review.
-
Dormant pathogenic CD4+ T cells are prevalent in the peripheral repertoire of healthy mice.Nat Commun. 2019 Oct 25;10(1):4882. doi: 10.1038/s41467-019-12820-3. Nat Commun. 2019. PMID: 31653839 Free PMC article.
-
Advances in the Study of Hyperprogression of Different Tumors Treated with PD-1/PD-L1 Antibody and the Mechanisms of Its Occurrence.Cancers (Basel). 2023 Feb 18;15(4):1314. doi: 10.3390/cancers15041314. Cancers (Basel). 2023. PMID: 36831655 Free PMC article. Review.
-
Laser Immunotherapy: A Potential Treatment Modality for Keratinocyte Carcinoma.Cancers (Basel). 2021 Oct 28;13(21):5405. doi: 10.3390/cancers13215405. Cancers (Basel). 2021. PMID: 34771568 Free PMC article. Review.
References
-
- Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–387. - PubMed
-
- Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446(7136):685–689. - PubMed
-
- Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. - PubMed
-
- Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

