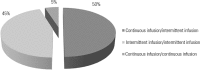Drug incompatibilities in the adult intensive care unit of a university hospital
- PMID: 27410410
- PMCID: PMC4943052
- DOI: 10.5935/0103-507X.20160029
Drug incompatibilities in the adult intensive care unit of a university hospital
Abstract
Objectives: This study sought to identify the physical and chemical incompatibilities among the drugs administered intravenously to patients admitted to an adult intensive care unit. We also aimed to establish pharmaceutical guidelines for administering incompatible drugs.
Methods: This cross-sectional, prospective, and quantitative study was conducted from July to September 2015. Drug incompatibilities were identified based on an analysis of the patient prescriptions available in the hospital online management system. A pharmaceutical intervention was performed using the guidelines on the preparation and administration of incompatible drugs. Adherence to those guidelines was subsequently assessed among the nursing staff.
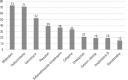
Results: A total of 100 prescriptions were analyzed; 68 were incompatible with the intravenous drugs prescribed. A total of 271 drug incompatibilities were found, averaging 4.0 ± 3.3 incompatibilities per prescription. The most commonly found drug incompatibilities were between midazolam and hydrocortisone (8.9%), between cefepime and midazolam (5.2%), and between hydrocortisone and vancomycin (5.2%). The drugs most commonly involved in incompatibilities were midazolam, hydrocortisone, and vancomycin. The most common incompatibilities occurred when a drug was administered via continuous infusion and another was administered intermittently (50%). Of the 68 prescriptions that led to pharmaceutical guidelines, 45 (66.2%) were fully adhered to by the nursing staff.
Conclusion: Patients under intensive care were subjected to a high rate of incompatibilities. Drug incompatibilities can be identified and eliminated by the pharmacist on the multidisciplinary team, thereby reducing undesirable effects among patients.
Objetivos: Identificar as incompatibilidades físico-químicas entre medicamentos administrados por via intravenosa em pacientes internados em um centro de tratamento intensivo adulto, bem como realizar orientações farmacêuticas para a administração de medicamentos incompatíveis.
Métodos: Estudo transversal, prospectivo, de caráter quantitativo, realizado no período de julho a setembro de 2015. As incompatibilidades foram identificadas a partir da análise das prescrições dos pacientes disponíveis no sistema on-line do hospital. Foi realizada uma intervenção farmacêutica por meio de orientações quanto à preparação e à administração dos medicamentos incompatíveis. Após, verificou-se a adesão dessas orientações por parte da equipe da enfermagem.
Resultados: Foram analisadas 100 prescrições; destas, 68 apresentaram incompatibilidade entre os medicamentos intravenosos prescritos. Foram encontradas 271 incompatibilidades, com média de 4,0 ± 3,3 incompatibilidades por prescrição. As incompatibilidades mais frequentes foram entre midazolam e hidrocortisona (8,9%), cefepime e midazolam (5,2%) e hidrocortisona e vancomicina (5,2%). Os medicamentos mais envolvidos em incompatibilidades foram o midazolam, a hidrocortisona e a vancomicina. As incompatibilidades foram mais frequentes entre um medicamento administrado por infusão contínua com outro de forma intermitente (50%). Das 68 prescrições que geraram orientação farmacêutica, 45 (66,2%) foram totalmente realizadas pela equipe de enfermagem.
Conclusão: Os pacientes em cuidados intensivos estiveram sujeitos a uma elevada ocorrência de incompatibilidades. As incompatibilidades medicamentosas podem ser identificadas e evitadas com a presença do farmacêutico na equipe multidisciplinar, diminuindo a ocorrência de efeitos indesejáveis ao paciente.
Conflict of interest statement
Figures
Similar articles
-
Incidence of intravenous drug incompatibilities in intensive care units.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015 Dec;159(4):652-6. doi: 10.5507/bp.2014.057. Epub 2014 Nov 6. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015. PMID: 25482735
-
Prevention of intravenous drug incompatibilities in an intensive care unit.Am J Health Syst Pharm. 2008 Oct 1;65(19):1834-40. doi: 10.2146/ajhp070633. Am J Health Syst Pharm. 2008. PMID: 18796425
-
Pharmacist recommendations in an intensive care unit: three-year clinical activities.Rev Bras Ter Intensiva. 2015 Apr-Jun;27(2):149-54. doi: 10.5935/0103-507X.20150026. Rev Bras Ter Intensiva. 2015. PMID: 26340155 Free PMC article.
-
Compatibility of drugs administered as Y-site infusion in intensive care units: A systematic review.Med Intensiva (Engl Ed). 2020 Mar;44(2):80-87. doi: 10.1016/j.medin.2018.08.004. Epub 2018 Sep 24. Med Intensiva (Engl Ed). 2020. PMID: 30262380 English, Spanish.
-
Drugs commonly administered by intravenous infusion in intensive care units: a practical guide.Crit Care Med. 1990 Feb;18(2):232-8. doi: 10.1097/00003246-199002000-00021. Crit Care Med. 1990. PMID: 2404666 Review. No abstract available.
Cited by
-
Harmonising IV Oxycodone with Paediatric Perioperative Medications: A Compatibility Study Through Y-Type Connectors.Drug Des Devel Ther. 2024 Mar 22;18:899-908. doi: 10.2147/DDDT.S444581. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38533429 Free PMC article.
-
Niosomes: A Strategy toward Prevention of Clinically Significant Drug Incompatibilities.Sci Rep. 2017 Jul 24;7(1):6340. doi: 10.1038/s41598-017-06955-w. Sci Rep. 2017. PMID: 28740102 Free PMC article.
-
Evaluation of Incompatible Coadministration of Continuous Intravenous Infusions in a Pediatric/Neonatal Intensive Care Unit.J Pediatr Pharmacol Ther. 2019 Nov-Dec;24(6):479-488. doi: 10.5863/1551-6776-24.6.479. J Pediatr Pharmacol Ther. 2019. PMID: 31719809 Free PMC article.
-
Y-Site Compatibility Studies of Ketoprofen with Parenteral Nutrition Admixtures for Central and Peripheral Administration.Pharmaceutics. 2022 Nov 23;14(12):2570. doi: 10.3390/pharmaceutics14122570. Pharmaceutics. 2022. PMID: 36559064 Free PMC article.
-
Avoiding incompatible drug pairs in central-venous catheters of patients receiving critical care: an algorithm-based analysis and a staff survey.Eur J Clin Pharmacol. 2023 Aug;79(8):1081-1089. doi: 10.1007/s00228-023-03509-0. Epub 2023 Jun 7. Eur J Clin Pharmacol. 2023. PMID: 37284873 Free PMC article.
References
-
- Secoli SR, Pérez-Esquirol E, de Las Heras-Matellán MJ, Vendrell-Bosh L, Ballarín-Alins E. Incompatibilities in intravenous therapy: What can be done to prevent them? Enferm Clin. 2009;19(6):349–353. Spanish. - PubMed
-
- Trissel LA. Handbook on injectable drugs. 17th ed. Maryland: American Society of Health- System Pharmacists; 2013.
-
- Kanji S, Lam J, Johanson C, Singh A, Goddard R, Fairbairn J, et al. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit Care Med. 2010;38(9):1890–1898. - PubMed
-
- Tatro DS. Drug interaction facts: the authority on drug interactions. St. Louis: Facts and Comparisons; 2006.
-
- Bentley J, Heard J, Collins G, Chung C. Mixing medicines: how to ensure patient safety. Pharmac J. 2015;294(7859)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical